 Found 170 hits Enz. Inhib. hit(s) with all data for entry = 50042717
Found 170 hits Enz. Inhib. hit(s) with all data for entry = 50042717 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429897
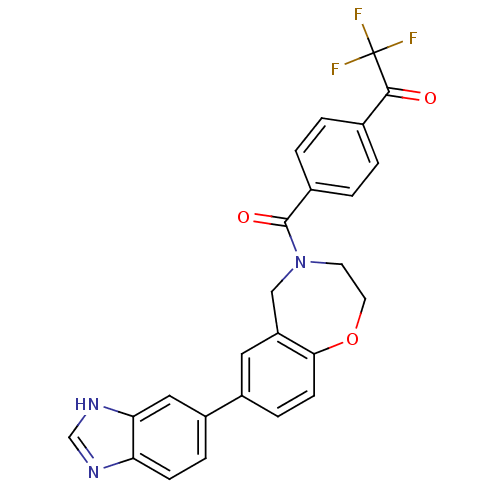
(CHEMBL2333379)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C25H18F3N3O3/c26-25(27,28)23(32)15-1-3-16(4-2-15)24(33)31-9-10-34-22-8-6-17(11-19(22)13-31)18-5-7-20-21(12-18)30-14-29-20/h1-8,11-12,14H,9-10,13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429896
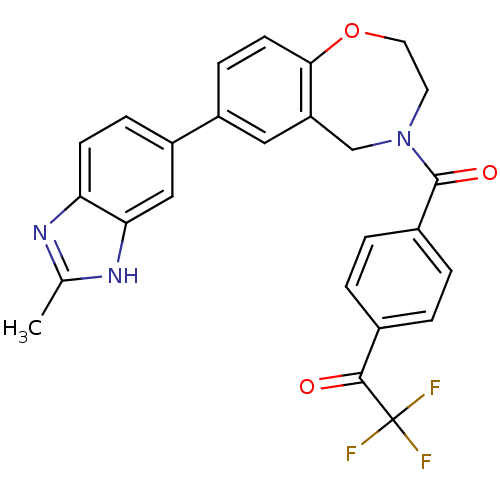
(CHEMBL2332918)Show SMILES Cc1nc2ccc(cc2[nH]1)-c1ccc2OCCN(Cc2c1)C(=O)c1ccc(cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H20F3N3O3/c1-15-30-21-8-6-19(13-22(21)31-15)18-7-9-23-20(12-18)14-32(10-11-35-23)25(34)17-4-2-16(3-5-17)24(33)26(27,28)29/h2-9,12-13H,10-11,14H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429895
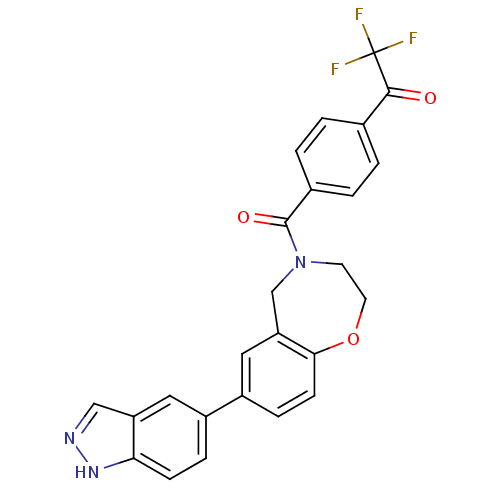
(CHEMBL2333375)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C25H18F3N3O3/c26-25(27,28)23(32)15-1-3-16(4-2-15)24(33)31-9-10-34-22-8-6-18(12-20(22)14-31)17-5-7-21-19(11-17)13-29-30-21/h1-8,11-13H,9-10,14H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429893

(CHEMBL2333373)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2OCCN(Cc2c1)C(=O)c1ccc(cc1)C(=O)C(F)(F)F Show InChI InChI=1S/C26H21F3N2O4/c1-16(32)30-22-4-2-3-19(14-22)20-9-10-23-21(13-20)15-31(11-12-35-23)25(34)18-7-5-17(6-8-18)24(33)26(27,28)29/h2-10,13-14H,11-12,15H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429892
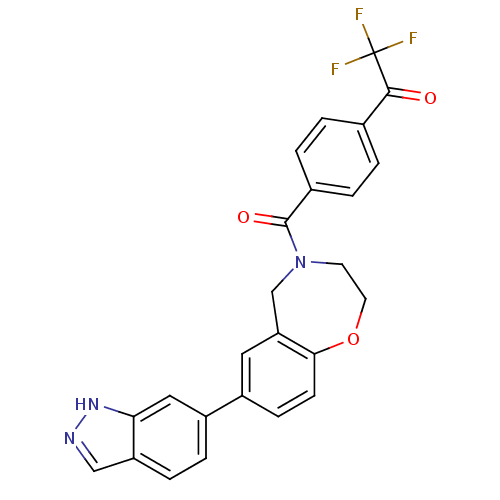
(CHEMBL2333374)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2cn[nH]c2c1 Show InChI InChI=1S/C25H18F3N3O3/c26-25(27,28)23(32)15-1-3-16(4-2-15)24(33)31-9-10-34-22-8-7-17(11-20(22)14-31)18-5-6-19-13-29-30-21(19)12-18/h1-8,11-13H,9-10,14H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429891
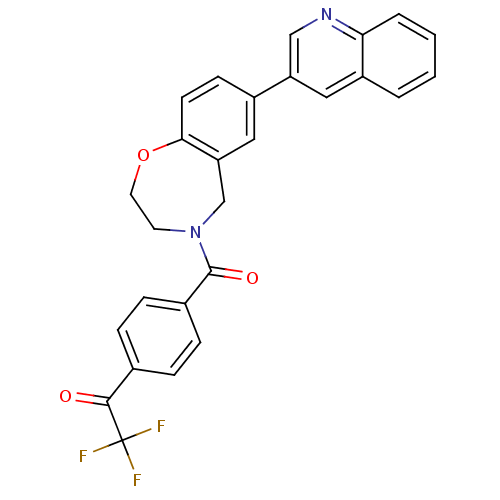
(CHEMBL2333366)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C27H19F3N2O3/c28-27(29,30)25(33)17-5-7-18(8-6-17)26(34)32-11-12-35-24-10-9-19(13-22(24)16-32)21-14-20-3-1-2-4-23(20)31-15-21/h1-10,13-15H,11-12,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429890
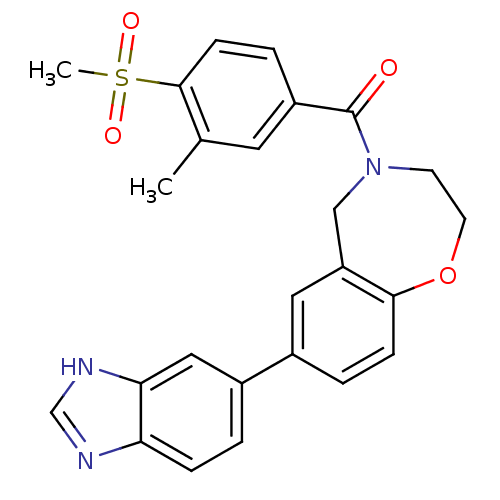
(CHEMBL2331575)Show SMILES Cc1cc(ccc1S(C)(=O)=O)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C25H23N3O4S/c1-16-11-19(5-8-24(16)33(2,30)31)25(29)28-9-10-32-23-7-4-17(12-20(23)14-28)18-3-6-21-22(13-18)27-15-26-21/h3-8,11-13,15H,9-10,14H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429889
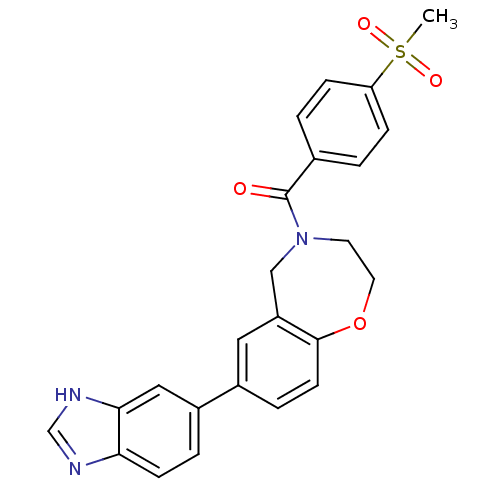
(CHEMBL2332922)Show SMILES CS(=O)(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C24H21N3O4S/c1-32(29,30)20-6-2-16(3-7-20)24(28)27-10-11-31-23-9-5-17(12-19(23)14-27)18-4-8-21-22(13-18)26-15-25-21/h2-9,12-13,15H,10-11,14H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429888
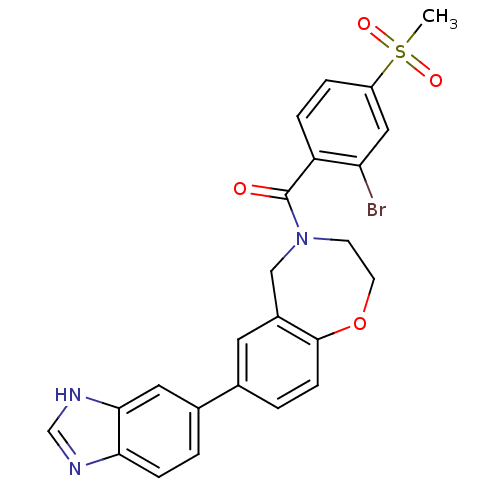
(CHEMBL2332929)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCOc3ccc(cc3C2)-c2ccc3nc[nH]c3c2)c(Br)c1 Show InChI InChI=1S/C24H20BrN3O4S/c1-33(30,31)18-4-5-19(20(25)12-18)24(29)28-8-9-32-23-7-3-15(10-17(23)13-28)16-2-6-21-22(11-16)27-14-26-21/h2-7,10-12,14H,8-9,13H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 693 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429887
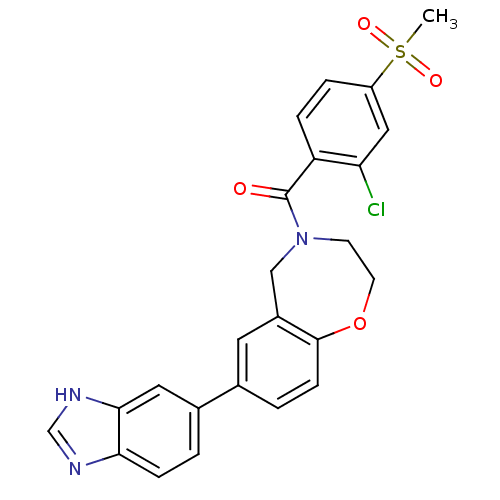
(CHEMBL2332928)Show SMILES CS(=O)(=O)c1ccc(C(=O)N2CCOc3ccc(cc3C2)-c2ccc3nc[nH]c3c2)c(Cl)c1 Show InChI InChI=1S/C24H20ClN3O4S/c1-33(30,31)18-4-5-19(20(25)12-18)24(29)28-8-9-32-23-7-3-15(10-17(23)13-28)16-2-6-21-22(11-16)27-14-26-21/h2-7,10-12,14H,8-9,13H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 705 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429886
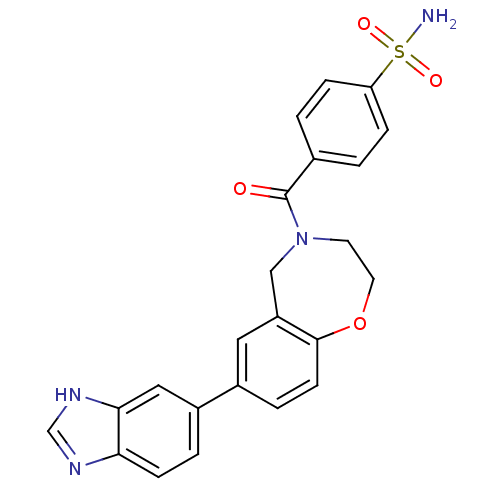
(CHEMBL2332920)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C23H20N4O4S/c24-32(29,30)19-5-1-15(2-6-19)23(28)27-9-10-31-22-8-4-16(11-18(22)13-27)17-3-7-20-21(12-17)26-14-25-20/h1-8,11-12,14H,9-10,13H2,(H,25,26)(H2,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 763 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429885

(CHEMBL2333378)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1cn[nH]c1 Show InChI InChI=1S/C21H16F3N3O3/c22-21(23,24)19(28)13-1-3-14(4-2-13)20(29)27-7-8-30-18-6-5-15(9-16(18)12-27)17-10-25-26-11-17/h1-6,9-11H,7-8,12H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429884
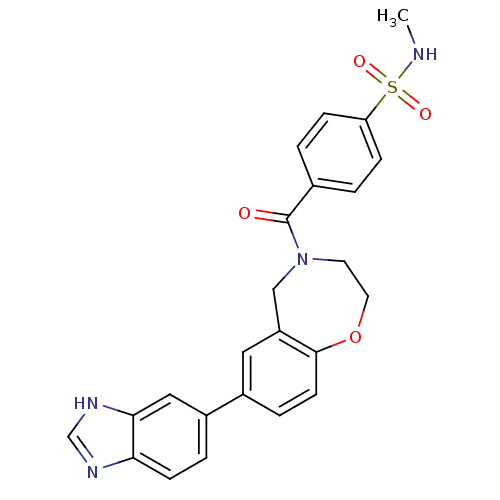
(CHEMBL2332921)Show SMILES CNS(=O)(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C24H22N4O4S/c1-25-33(30,31)20-6-2-16(3-7-20)24(29)28-10-11-32-23-9-5-17(12-19(23)14-28)18-4-8-21-22(13-18)27-15-26-21/h2-9,12-13,15,25H,10-11,14H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429883
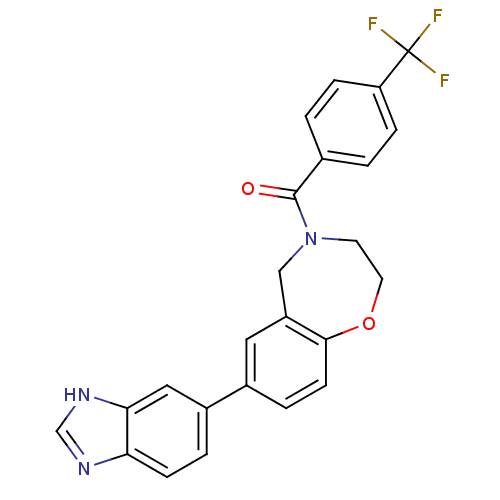
(CHEMBL2332919)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C24H18F3N3O2/c25-24(26,27)19-5-1-15(2-6-19)23(31)30-9-10-32-22-8-4-16(11-18(22)13-30)17-3-7-20-21(12-17)29-14-28-20/h1-8,11-12,14H,9-10,13H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429882
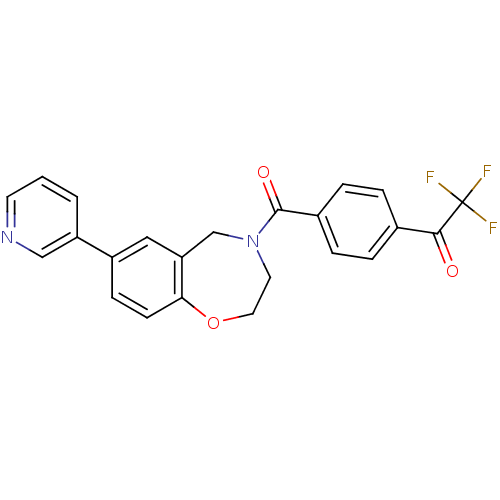
(CHEMBL2333372)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1cccnc1 Show InChI InChI=1S/C23H17F3N2O3/c24-23(25,26)21(29)15-3-5-16(6-4-15)22(30)28-10-11-31-20-8-7-17(12-19(20)14-28)18-2-1-9-27-13-18/h1-9,12-13H,10-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429881
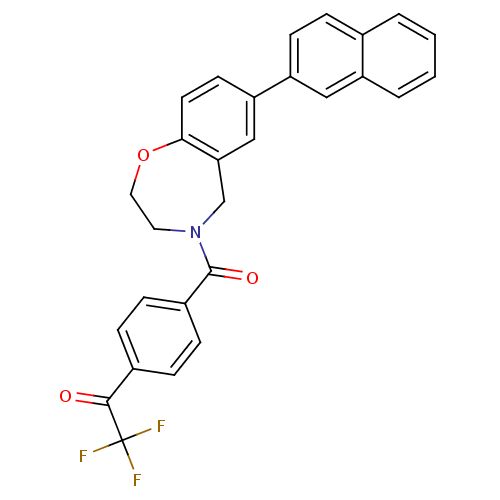
(CHEMBL2333370)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C28H20F3NO3/c29-28(30,31)26(33)19-6-8-20(9-7-19)27(34)32-13-14-35-25-12-11-23(16-24(25)17-32)22-10-5-18-3-1-2-4-21(18)15-22/h1-12,15-16H,13-14,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429880
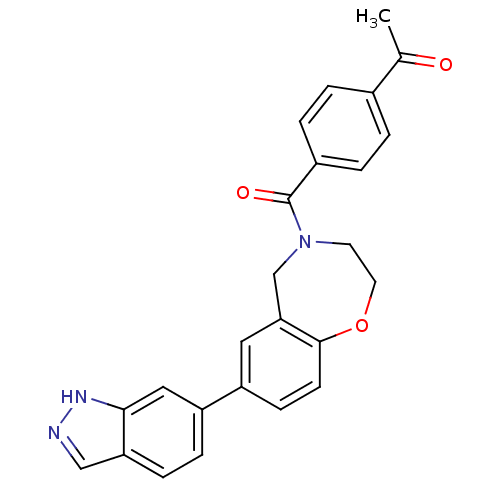
(CHEMBL2333376)Show SMILES CC(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2cn[nH]c2c1 Show InChI InChI=1S/C25H21N3O3/c1-16(29)17-2-4-18(5-3-17)25(30)28-10-11-31-24-9-8-19(12-22(24)15-28)20-6-7-21-14-26-27-23(21)13-20/h2-9,12-14H,10-11,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429879
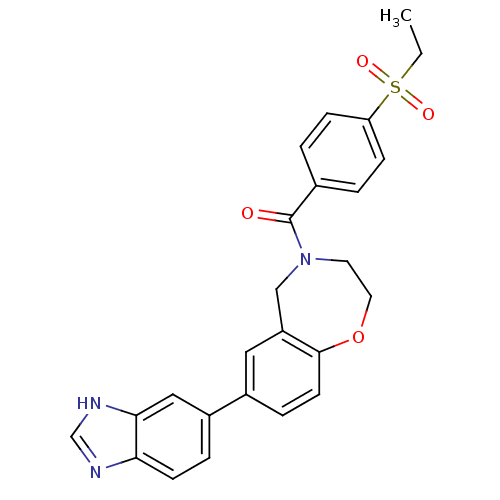
(CHEMBL2332923)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C25H23N3O4S/c1-2-33(30,31)21-7-3-17(4-8-21)25(29)28-11-12-32-24-10-6-18(13-20(24)15-28)19-5-9-22-23(14-19)27-16-26-22/h3-10,13-14,16H,2,11-12,15H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429878
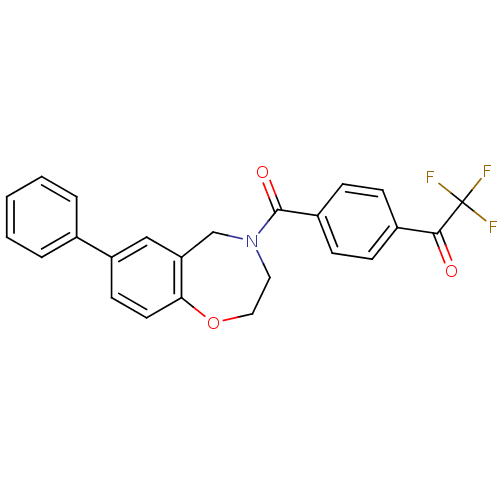
(CHEMBL2333371)Show SMILES FC(F)(F)C(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccccc1 Show InChI InChI=1S/C24H18F3NO3/c25-24(26,27)22(29)17-6-8-18(9-7-17)23(30)28-12-13-31-21-11-10-19(14-20(21)15-28)16-4-2-1-3-5-16/h1-11,14H,12-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429877

(CHEMBL2333362)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C25H22FN3O4S/c1-15-19(5-8-23(24(15)26)34(2,31)32)25(30)29-9-10-33-22-7-4-16(11-18(22)13-29)17-3-6-20-21(12-17)28-14-27-20/h3-8,11-12,14H,9-10,13H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429876
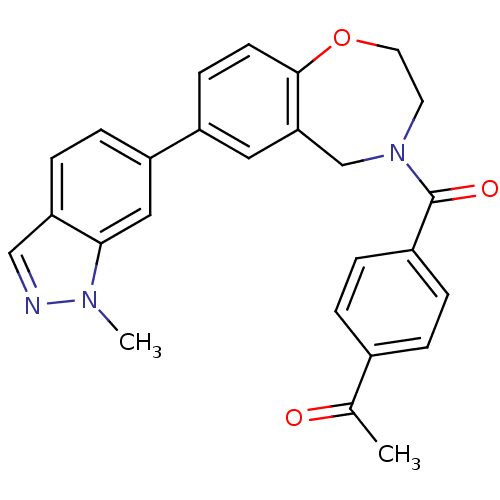
(CHEMBL2333377)Show SMILES CC(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc2cnn(C)c2c1 Show InChI InChI=1S/C26H23N3O3/c1-17(30)18-3-5-19(6-4-18)26(31)29-11-12-32-25-10-9-20(13-23(25)16-29)21-7-8-22-15-27-28(2)24(22)14-21/h3-10,13-15H,11-12,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429875
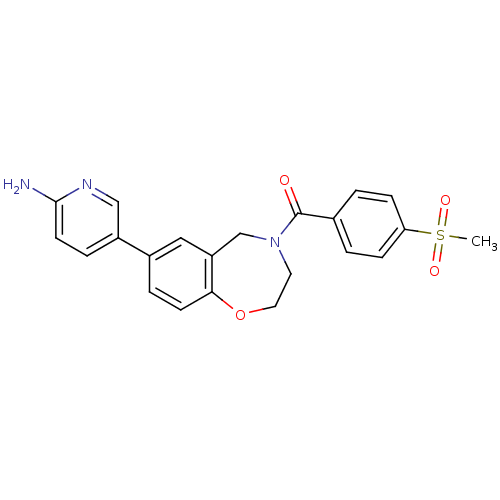
(CHEMBL2333364)Show SMILES CS(=O)(=O)c1ccc(cc1)C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1 Show InChI InChI=1S/C22H21N3O4S/c1-30(27,28)19-6-2-15(3-7-19)22(26)25-10-11-29-20-8-4-16(12-18(20)14-25)17-5-9-21(23)24-13-17/h2-9,12-13H,10-11,14H2,1H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429872
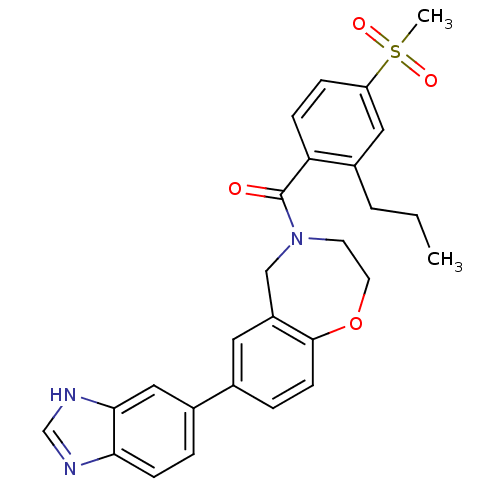
(CHEMBL2332926)Show SMILES CCCc1cc(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C27H27N3O4S/c1-3-4-20-14-22(35(2,32)33)7-8-23(20)27(31)30-11-12-34-26-10-6-18(13-21(26)16-30)19-5-9-24-25(15-19)29-17-28-24/h5-10,13-15,17H,3-4,11-12,16H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429871
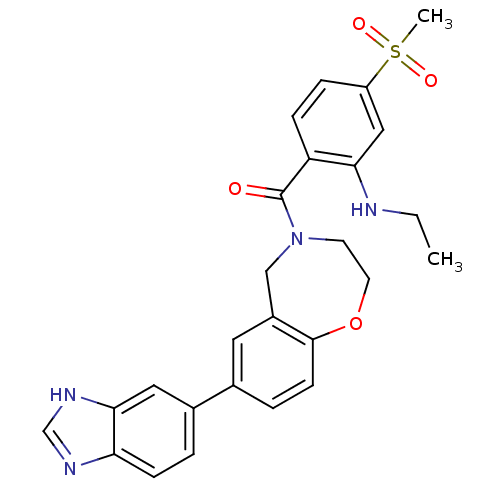
(CHEMBL2332927)Show SMILES CCNc1cc(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C26H26N4O4S/c1-3-27-23-14-20(35(2,32)33)6-7-21(23)26(31)30-10-11-34-25-9-5-17(12-19(25)15-30)18-4-8-22-24(13-18)29-16-28-22/h4-9,12-14,16,27H,3,10-11,15H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429870
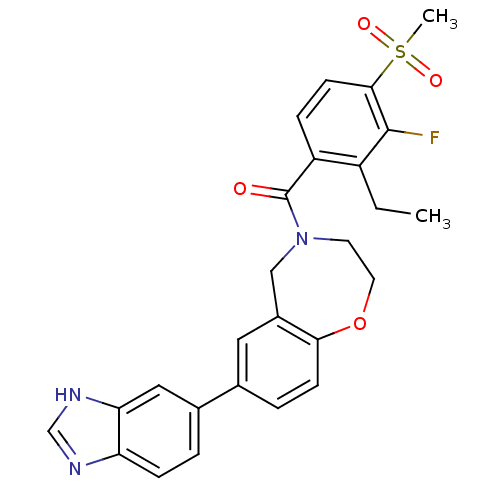
(CHEMBL2333363)Show SMILES CCc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C26H24FN3O4S/c1-3-19-20(6-9-24(25(19)27)35(2,32)33)26(31)30-10-11-34-23-8-5-16(12-18(23)14-30)17-4-7-21-22(13-17)29-15-28-21/h4-9,12-13,15H,3,10-11,14H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429873

(CHEMBL2332925)Show SMILES CCc1cc(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C26H25N3O4S/c1-3-17-13-21(34(2,31)32)6-7-22(17)26(30)29-10-11-33-25-9-5-18(12-20(25)15-29)19-4-8-23-24(14-19)28-16-27-23/h4-9,12-14,16H,3,10-11,15H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429874

(CHEMBL2332924)Show SMILES Cc1cc(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc2nc[nH]c2c1)S(C)(=O)=O Show InChI InChI=1S/C25H23N3O4S/c1-16-11-20(33(2,30)31)5-6-21(16)25(29)28-9-10-32-24-8-4-17(12-19(24)14-28)18-3-7-22-23(13-18)27-15-26-22/h3-8,11-13,15H,9-10,14H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50429894
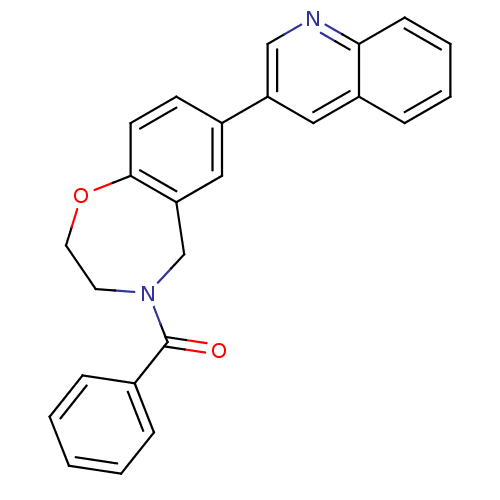
(CHEMBL2333369)Show SMILES O=C(N1CCOc2ccc(cc2C1)-c1cnc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C25H20N2O2/c28-25(18-6-2-1-3-7-18)27-12-13-29-24-11-10-19(14-22(24)17-27)21-15-20-8-4-5-9-23(20)26-16-21/h1-11,14-16H,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate after 2 hrs by luminescence assay |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of cRAF (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of ERBB2 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of FLT1 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of KIT (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of MET (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50429867

(CHEMBL2333365)Show SMILES Cc1c(F)c(ccc1C(=O)N1CCOc2ccc(cc2C1)-c1ccc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C23H22FN3O4S/c1-14-18(5-7-20(22(14)24)32(2,29)30)23(28)27-9-10-31-19-6-3-15(11-17(19)13-27)16-4-8-21(25)26-12-16/h3-8,11-12H,9-10,13H2,1-2H3,(H2,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of CDC7 (unknown origin) |
J Med Chem 56: 2218-34 (2013)
Article DOI: 10.1021/jm3007933
BindingDB Entry DOI: 10.7270/Q2ZC847X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data