 Found 43 hits Enz. Inhib. hit(s) with all data for entry = 50043011
Found 43 hits Enz. Inhib. hit(s) with all data for entry = 50043011 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434486

(CHEMBL2385548)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C34H28N6O2/c35-18-23-5-7-24(8-6-23)32-17-27-15-26-16-29(39-13-11-36-12-14-39)9-10-31(26)40(34(41)33(27)42-32)21-25-3-1-2-4-30(25)28-19-37-22-38-20-28/h1-10,16-17,19-20,22,36H,11-15,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434488
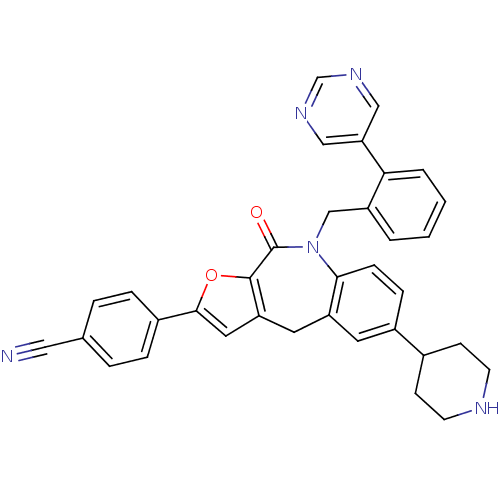
(CHEMBL2385549)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)C1CCNCC1 Show InChI InChI=1S/C35H29N5O2/c36-18-23-5-7-25(8-6-23)33-17-29-16-28-15-26(24-11-13-37-14-12-24)9-10-32(28)40(35(41)34(29)42-33)21-27-3-1-2-4-31(27)30-19-38-22-39-20-30/h1-10,15,17,19-20,22,24,37H,11-14,16,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434494

(CHEMBL2385542)Show SMILES COc1cncc(c1)-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H31N5O3/c1-43-31-18-29(21-39-22-31)32-5-3-2-4-26(32)23-41-33-11-10-30(40-14-12-38-13-15-40)17-27(33)16-28-19-34(44-35(28)36(41)42)25-8-6-24(20-37)7-9-25/h2-11,17-19,21-22,38H,12-16,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434489
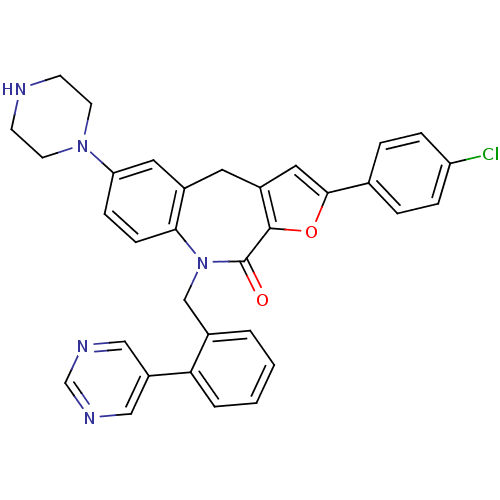
(CHEMBL2385547)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cncnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C33H28ClN5O2/c34-27-7-5-22(6-8-27)31-17-25-15-24-16-28(38-13-11-35-12-14-38)9-10-30(24)39(33(40)32(25)41-31)20-23-3-1-2-4-29(23)26-18-36-21-37-19-26/h1-10,16-19,21,35H,11-15,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434490
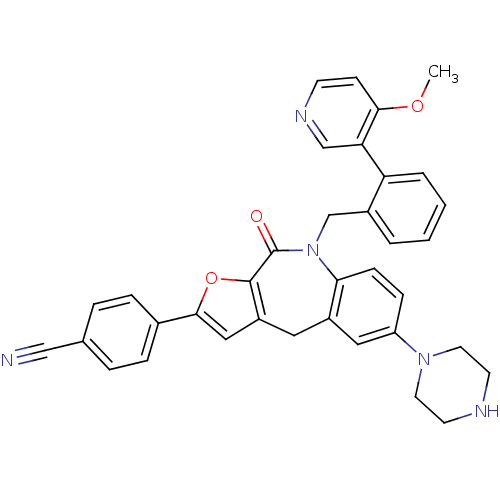
(CHEMBL2385546)Show SMILES COc1ccncc1-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H31N5O3/c1-43-33-12-13-39-22-31(33)30-5-3-2-4-26(30)23-41-32-11-10-29(40-16-14-38-15-17-40)19-27(32)18-28-20-34(44-35(28)36(41)42)25-8-6-24(21-37)7-9-25/h2-13,19-20,22,38H,14-18,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434491
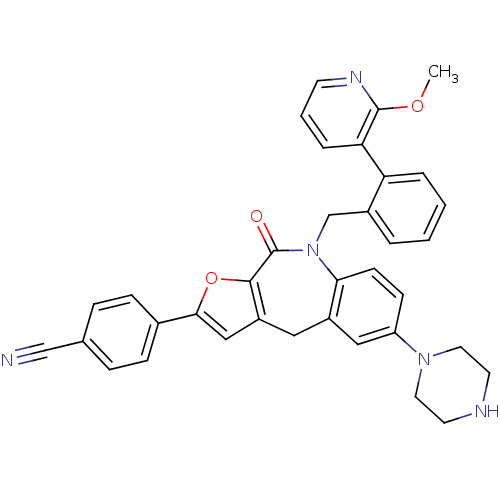
(CHEMBL2385545)Show SMILES COc1ncccc1-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H31N5O3/c1-43-35-31(7-4-14-39-35)30-6-3-2-5-26(30)23-41-32-13-12-29(40-17-15-38-16-18-40)20-27(32)19-28-21-33(44-34(28)36(41)42)25-10-8-24(22-37)9-11-25/h2-14,20-21,38H,15-19,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434487

(CHEMBL2385550)Show SMILES FC1(F)c2cc(oc2C(=O)N(Cc2ccccc2-c2cncnc2)c2ccc(cc12)C1CCNCC1)-c1ccc(cc1)C#N Show InChI InChI=1S/C35H27F2N5O2/c36-35(37)29-15-25(23-11-13-39-14-12-23)9-10-31(29)42(20-26-3-1-2-4-28(26)27-18-40-21-41-19-27)34(43)33-30(35)16-32(44-33)24-7-5-22(17-38)6-8-24/h1-10,15-16,18-19,21,23,39H,11-14,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434504
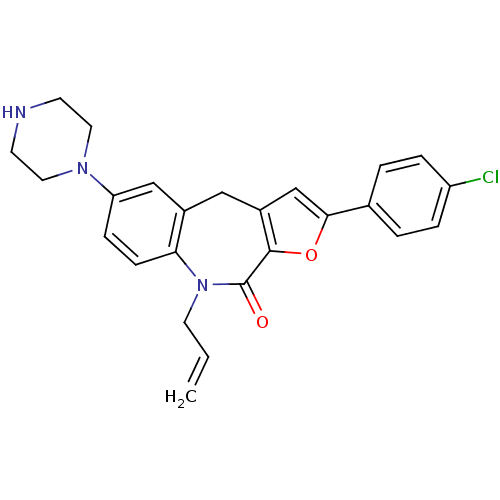
(CHEMBL2385532)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(CC=C)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C25H24ClN3O2/c1-2-11-29-22-8-7-21(28-12-9-27-10-13-28)15-18(22)14-19-16-23(31-24(19)25(29)30)17-3-5-20(26)6-4-17/h2-8,15-16,27H,1,9-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434496

(CHEMBL2385540)Show SMILES Fc1cncc(c1)-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C35H28FN5O2/c36-29-16-28(20-39-21-29)31-4-2-1-3-25(31)22-41-32-10-9-30(40-13-11-38-12-14-40)17-26(32)15-27-18-33(43-34(27)35(41)42)24-7-5-23(19-37)6-8-24/h1-10,16-18,20-21,38H,11-15,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434499
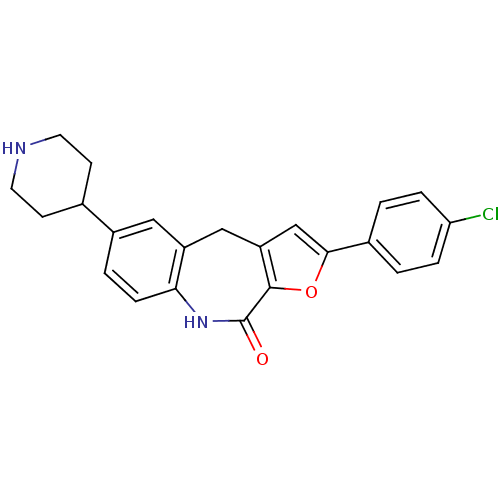
(CHEMBL2385537)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3NC(=O)c2o1)C1CCNCC1 Show InChI InChI=1S/C23H21ClN2O2/c24-19-4-1-15(2-5-19)21-13-18-12-17-11-16(14-7-9-25-10-8-14)3-6-20(17)26-23(27)22(18)28-21/h1-6,11,13-14,25H,7-10,12H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434492
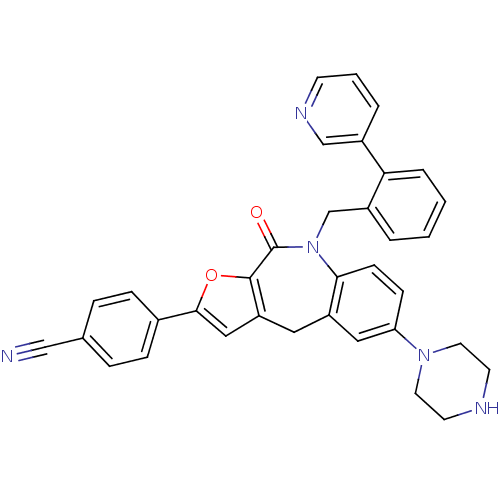
(CHEMBL2385544)Show SMILES O=C1N(Cc2ccccc2-c2cccnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C35H29N5O2/c36-21-24-7-9-25(10-8-24)33-20-29-18-28-19-30(39-16-14-37-15-17-39)11-12-32(28)40(35(41)34(29)42-33)23-27-4-1-2-6-31(27)26-5-3-13-38-22-26/h1-13,19-20,22,37H,14-18,23H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434495
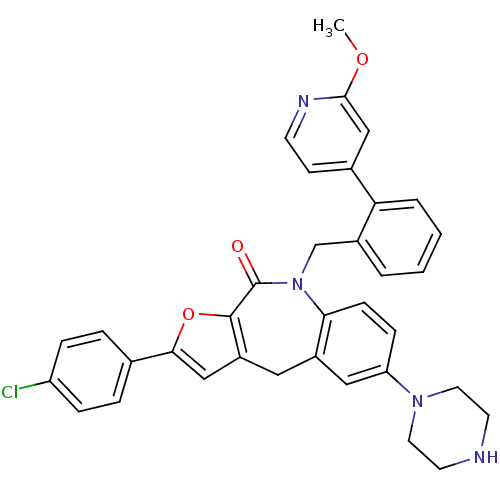
(CHEMBL2385541)Show SMILES COc1cc(ccn1)-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(Cl)cc1)N1CCNCC1 Show InChI InChI=1S/C35H31ClN4O3/c1-42-33-21-24(12-13-38-33)30-5-3-2-4-25(30)22-40-31-11-10-29(39-16-14-37-15-17-39)19-26(31)18-27-20-32(43-34(27)35(40)41)23-6-8-28(36)9-7-23/h2-13,19-21,37H,14-18,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434485
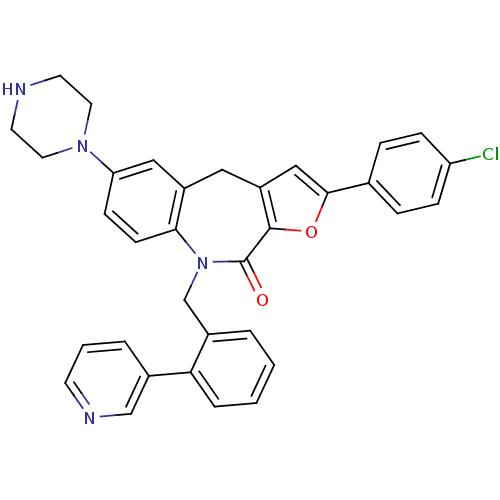
(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434498

(CHEMBL2385538)Show SMILES O=C1Nc2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)C1CCNCC1 Show InChI InChI=1S/C24H21N3O2/c25-14-15-1-3-17(4-2-15)22-13-20-12-19-11-18(16-7-9-26-10-8-16)5-6-21(19)27-24(28)23(20)29-22/h1-6,11,13,16,26H,7-10,12H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434493
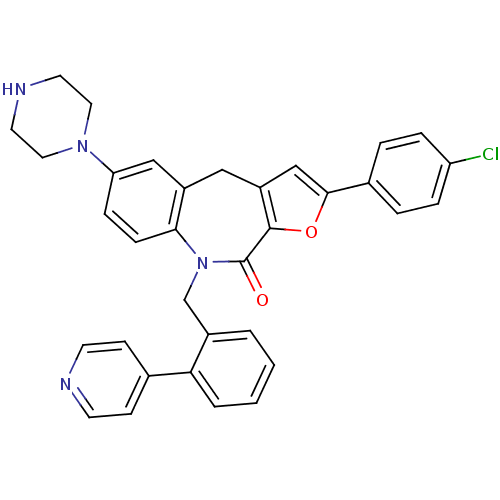
(CHEMBL2385085)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3ccncc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-7-5-24(6-8-28)32-21-27-19-26-20-29(38-17-15-37-16-18-38)9-10-31(26)39(34(40)33(27)41-32)22-25-3-1-2-4-30(25)23-11-13-36-14-12-23/h1-14,20-21,37H,15-19,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434500
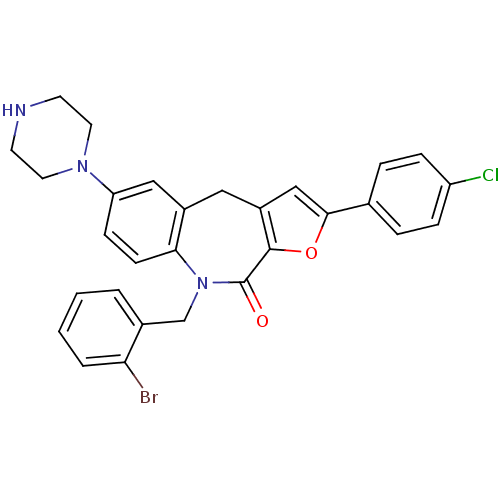
(CHEMBL2385536)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3Br)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C29H25BrClN3O2/c30-25-4-2-1-3-20(25)18-34-26-10-9-24(33-13-11-32-12-14-33)16-21(26)15-22-17-27(36-28(22)29(34)35)19-5-7-23(31)8-6-19/h1-10,16-17,32H,11-15,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434501
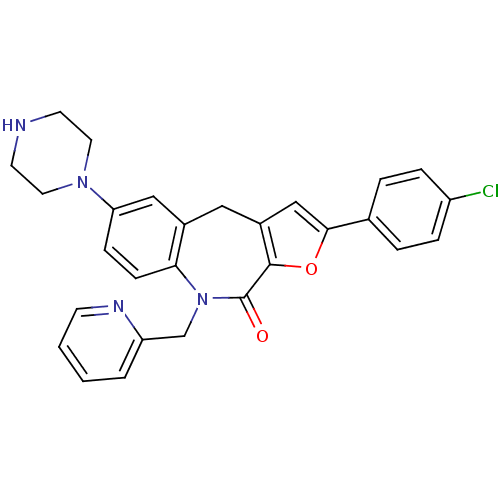
(CHEMBL2385535)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccn3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C28H25ClN4O2/c29-22-6-4-19(5-7-22)26-17-21-15-20-16-24(32-13-11-30-12-14-32)8-9-25(20)33(28(34)27(21)35-26)18-23-3-1-2-10-31-23/h1-10,16-17,30H,11-15,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434497
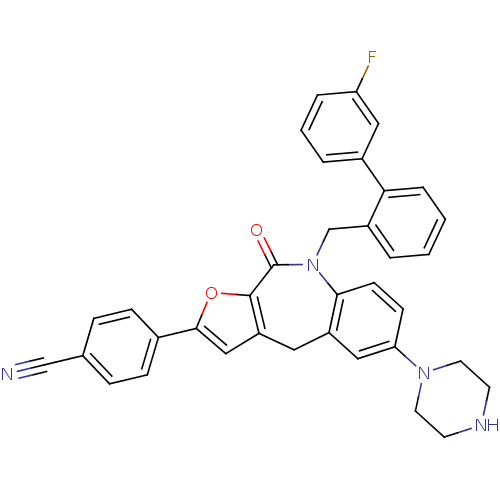
(CHEMBL2385539)Show SMILES Fc1cccc(c1)-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H29FN4O2/c37-30-6-3-5-26(19-30)32-7-2-1-4-27(32)23-41-33-13-12-31(40-16-14-39-15-17-40)20-28(33)18-29-21-34(43-35(29)36(41)42)25-10-8-24(22-38)9-11-25/h1-13,19-21,39H,14-18,23H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434505
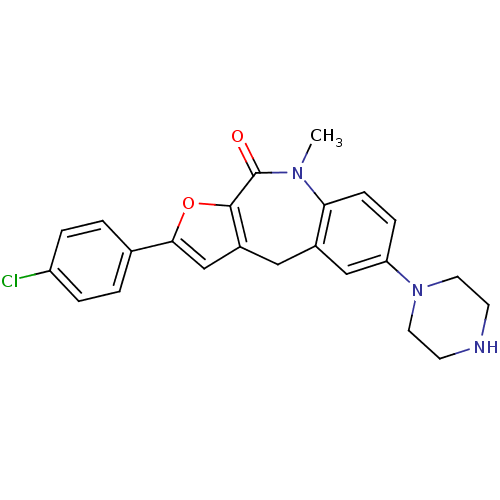
(CHEMBL2385531)Show SMILES CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(Cl)cc1)N1CCNCC1 Show InChI InChI=1S/C23H22ClN3O2/c1-26-20-7-6-19(27-10-8-25-9-11-27)13-16(20)12-17-14-21(29-22(17)23(26)28)15-2-4-18(24)5-3-15/h2-7,13-14,25H,8-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362170

(CHEMBL1938682)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3NC(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C22H20ClN3O2/c23-17-3-1-14(2-4-17)20-13-16-11-15-12-18(26-9-7-24-8-10-26)5-6-19(15)25-22(27)21(16)28-20/h1-6,12-13,24H,7-11H2,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434502

(CHEMBL2385534)Show SMILES FC(F)CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(Cl)cc1)N1CCNCC1 Show InChI InChI=1S/C24H22ClF2N3O2/c25-18-3-1-15(2-4-18)21-13-17-11-16-12-19(29-9-7-28-8-10-29)5-6-20(16)30(14-22(26)27)24(31)23(17)32-21/h1-6,12-13,22,28H,7-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434503

(CHEMBL2385533)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(CC3CC3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C26H26ClN3O2/c27-21-5-3-18(4-6-21)24-15-20-13-19-14-22(29-11-9-28-10-12-29)7-8-23(19)30(16-17-1-2-17)26(31)25(20)32-24/h3-8,14-15,17,28H,1-2,9-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362171
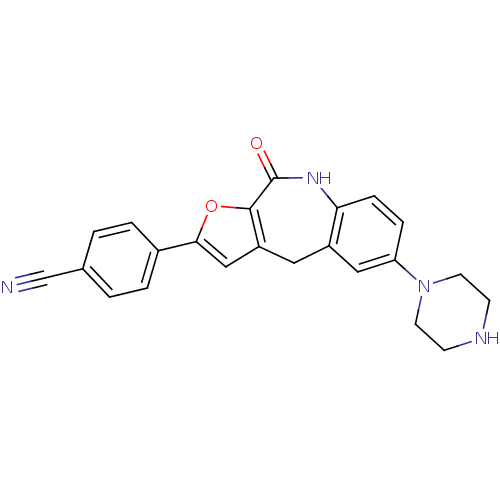
(CHEMBL1938683)Show SMILES O=C1Nc2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C23H20N4O2/c24-14-15-1-3-16(4-2-15)21-13-18-11-17-12-19(27-9-7-25-8-10-27)5-6-20(17)26-23(28)22(18)29-21/h1-6,12-13,25H,7-11H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380305
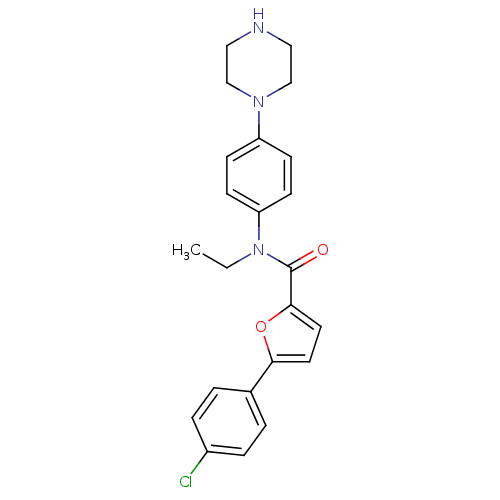
(CHEMBL2017459)Show SMILES CCN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C23H24ClN3O2/c1-2-27(20-9-7-19(8-10-20)26-15-13-25-14-16-26)23(28)22-12-11-21(29-22)17-3-5-18(24)6-4-17/h3-12,25H,2,13-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50380304
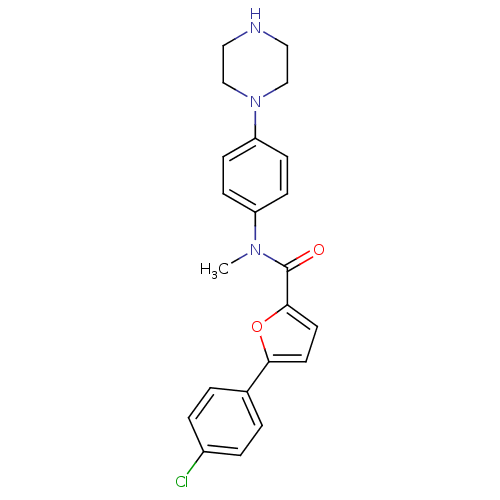
(CHEMBL2017458)Show SMILES CN(C(=O)c1ccc(o1)-c1ccc(Cl)cc1)c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C22H22ClN3O2/c1-25(18-6-8-19(9-7-18)26-14-12-24-13-15-26)22(27)21-11-10-20(28-21)16-2-4-17(23)5-3-16/h2-11,24H,12-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362169
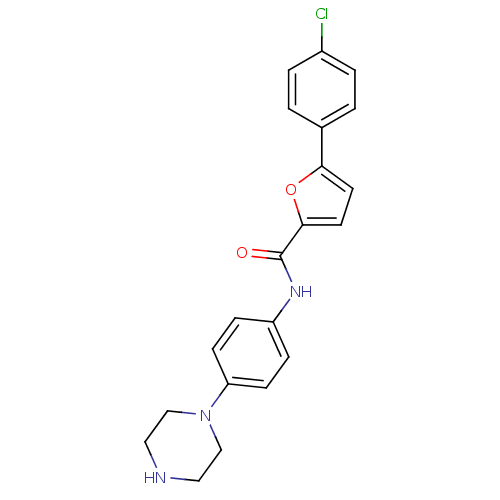
(CHEMBL1938680)Show SMILES Clc1ccc(cc1)-c1ccc(o1)C(=O)Nc1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2/c22-16-3-1-15(2-4-16)19-9-10-20(27-19)21(26)24-17-5-7-18(8-6-17)25-13-11-23-12-14-25/h1-10,23H,11-14H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of NEK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKKB (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IRAK4 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type IV
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CAMK4 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TSSK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MET (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CSNK1D (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50434485

(CHEMBL2385543)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cccnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C34H29ClN4O2/c35-28-9-7-23(8-10-28)32-20-27-18-26-19-29(38-16-14-36-15-17-38)11-12-31(26)39(34(40)33(27)41-32)22-25-4-1-2-6-30(25)24-5-3-13-37-21-24/h1-13,19-21,36H,14-18,22H2 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PLK3 (unknown origin) |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434486

(CHEMBL2385548)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C34H28N6O2/c35-18-23-5-7-24(8-6-23)32-17-27-15-26-16-29(39-13-11-36-12-14-39)9-10-31(26)40(34(41)33(27)42-32)21-25-3-1-2-4-30(25)28-19-37-22-38-20-28/h1-10,16-17,19-20,22,36H,11-15,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 138 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 in human THP1 cells assessed as phosphorylation of HSP27 at serine78 after 60 mins |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data