 Found 29 hits Enz. Inhib. hit(s) with all data for entry = 50043567
Found 29 hits Enz. Inhib. hit(s) with all data for entry = 50043567 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse AChE |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM31904
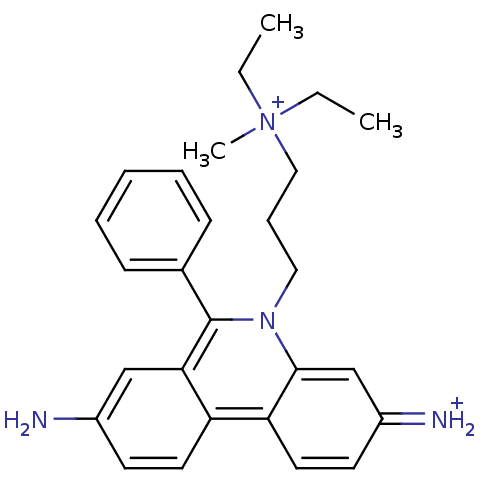
(CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...)Show SMILES CC[N+](C)(CC)CCCn1c(-c2ccccc2)c2cc(N)ccc2c2ccc(=[NH2+])cc12 Show InChI InChI=1S/C27H33N4/c1-4-31(3,5-2)17-9-16-30-26-19-22(29)13-15-24(26)23-14-12-21(28)18-25(23)27(30)20-10-7-6-8-11-20/h6-8,10-15,18-19,29H,4-5,9,16-17,28H2,1-3H3/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of wild type mouse AChE expressed in HEK293 cells using ATCh as substrate |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443031

(CHEMBL3087804)Show SMILES CCN(CC)CCNS(=O)(=O)c1ccc(Oc2c(Cl)cccc2[N+]([O-])=O)cc1 Show InChI InChI=1S/C18H22ClN3O5S/c1-3-21(4-2)13-12-20-28(25,26)15-10-8-14(9-11-15)27-18-16(19)6-5-7-17(18)22(23)24/h5-11,20H,3-4,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443045
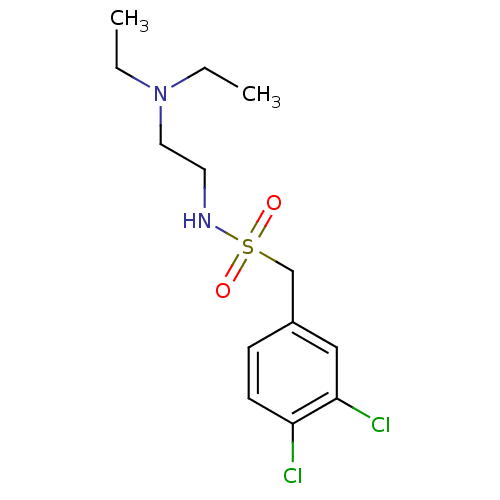
(CHEMBL3087811)Show InChI InChI=1S/C13H20Cl2N2O2S/c1-3-17(4-2)8-7-16-20(18,19)10-11-5-6-12(14)13(15)9-11/h5-6,9,16H,3-4,7-8,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443042

(CHEMBL3087814)Show InChI InChI=1S/C13H22N2O3S/c1-4-15(5-2)10-9-14-19(16,17)13-8-6-7-12(11-13)18-3/h6-8,11,14H,4-5,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443046

(CHEMBL3087810)Show InChI InChI=1S/C13H21BrN2O2S/c1-3-16(4-2)10-9-15-19(17,18)11-12-5-7-13(14)8-6-12/h5-8,15H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443029
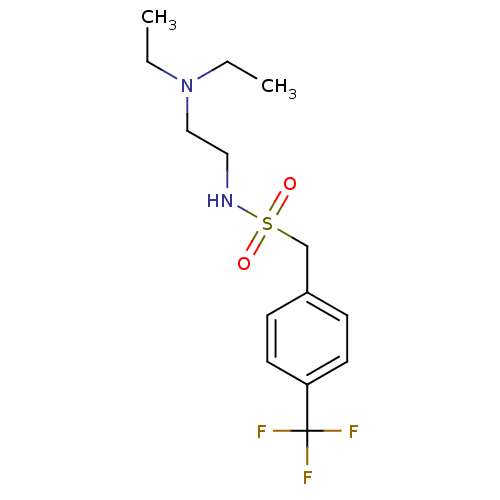
(CHEMBL3087809)Show InChI InChI=1S/C14H21F3N2O2S/c1-3-19(4-2)10-9-18-22(20,21)11-12-5-7-13(8-6-12)14(15,16)17/h5-8,18H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443030
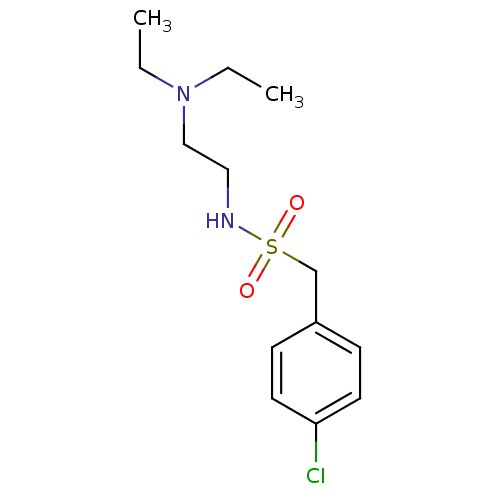
(CHEMBL3087808)Show InChI InChI=1S/C13H21ClN2O2S/c1-3-16(4-2)10-9-15-19(17,18)11-12-5-7-13(14)8-6-12/h5-8,15H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443027
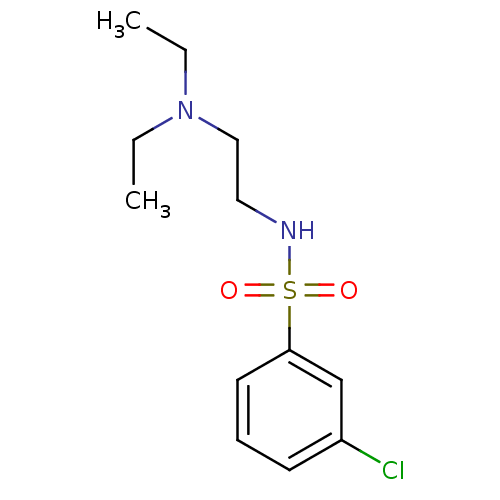
(CHEMBL3087794)Show InChI InChI=1S/C12H19ClN2O2S/c1-3-15(4-2)9-8-14-18(16,17)12-7-5-6-11(13)10-12/h5-7,10,14H,3-4,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443028
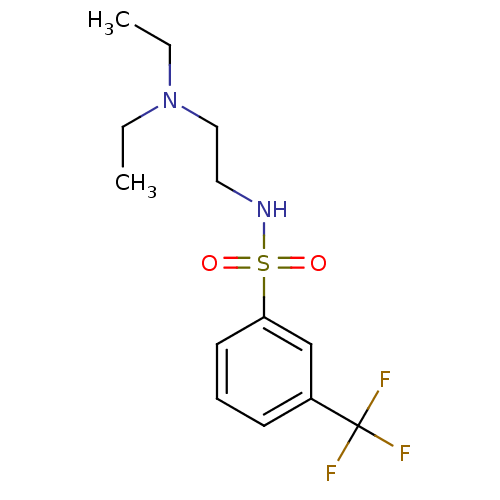
(CHEMBL3087815)Show InChI InChI=1S/C13H19F3N2O2S/c1-3-18(4-2)9-8-17-21(19,20)12-7-5-6-11(10-12)13(14,15)16/h5-7,10,17H,3-4,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443044

(CHEMBL3087812)Show InChI InChI=1S/C12H20N2O2S/c1-3-14(4-2)11-10-13-17(15,16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443047

(CHEMBL3087807)Show InChI InChI=1S/C13H21FN2O2S/c1-3-16(4-2)10-9-15-19(17,18)11-12-5-7-13(14)8-6-12/h5-8,15H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443043

(CHEMBL3087813)Show InChI InChI=1S/C12H19FN2O2S/c1-3-15(4-2)10-9-14-18(16,17)12-8-6-5-7-11(12)13/h5-8,14H,3-4,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443034

(CHEMBL3087801)Show InChI InChI=1S/C16H22N2O2S/c1-3-18(4-2)13-12-17-21(19,20)16-11-7-9-14-8-5-6-10-15(14)16/h5-11,17H,3-4,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443048
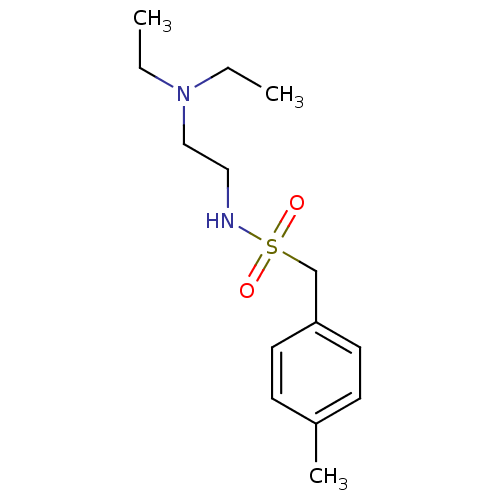
(CHEMBL3087806)Show InChI InChI=1S/C14H24N2O2S/c1-4-16(5-2)11-10-15-19(17,18)12-14-8-6-13(3)7-9-14/h6-9,15H,4-5,10-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443035

(CHEMBL3087800)Show InChI InChI=1S/C12H18F2N2O2S/c1-3-16(4-2)8-7-15-19(17,18)10-5-6-11(13)12(14)9-10/h5-6,9,15H,3-4,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443039

(CHEMBL3087797)Show InChI InChI=1S/C12H19FN2O2S/c1-3-15(4-2)10-9-14-18(16,17)12-7-5-11(13)6-8-12/h5-8,14H,3-4,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443038

(CHEMBL1624646)Show InChI InChI=1S/C12H19ClN2O2S/c1-3-15(4-2)10-9-14-18(16,17)12-7-5-11(13)6-8-12/h5-8,14H,3-4,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443036

(CHEMBL3087799)Show InChI InChI=1S/C12H18ClFN2O2S/c1-3-16(4-2)8-7-15-19(17,18)12-6-5-10(13)9-11(12)14/h5-6,9,15H,3-4,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443037

(CHEMBL3087798)Show InChI InChI=1S/C12H19N3O4S/c1-3-14(4-2)10-9-13-20(18,19)12-7-5-11(6-8-12)15(16)17/h5-8,13H,3-4,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443041

(CHEMBL3087795)Show InChI InChI=1S/C13H22N2O3S/c1-4-15(5-2)11-10-14-19(16,17)13-8-6-12(18-3)7-9-13/h6-9,14H,4-5,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443040
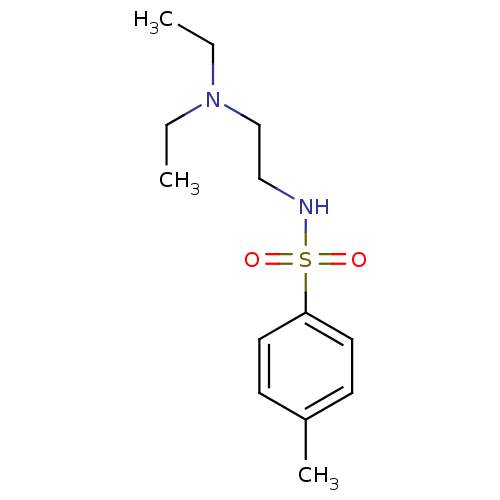
(CHEMBL3087796)Show InChI InChI=1S/C13H22N2O2S/c1-4-15(5-2)11-10-14-18(16,17)13-8-6-12(3)7-9-13/h6-9,14H,4-5,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443049

(CHEMBL3087805)Show InChI InChI=1S/C13H22N2O2S/c1-3-15(4-2)11-10-14-18(16,17)12-13-8-6-5-7-9-13/h5-9,14H,3-4,10-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443032
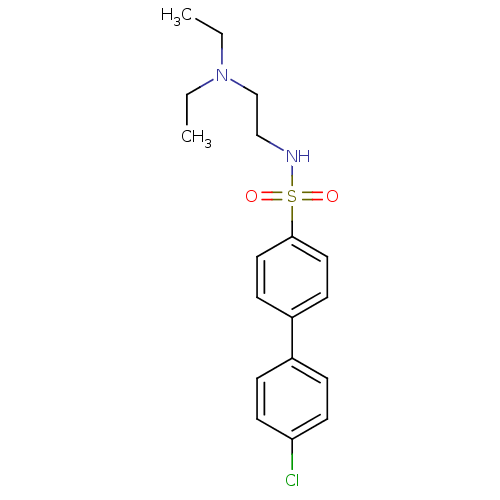
(CHEMBL3087803)Show InChI InChI=1S/C18H23ClN2O2S/c1-3-21(4-2)14-13-20-24(22,23)18-11-7-16(8-12-18)15-5-9-17(19)10-6-15/h5-12,20H,3-4,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443033
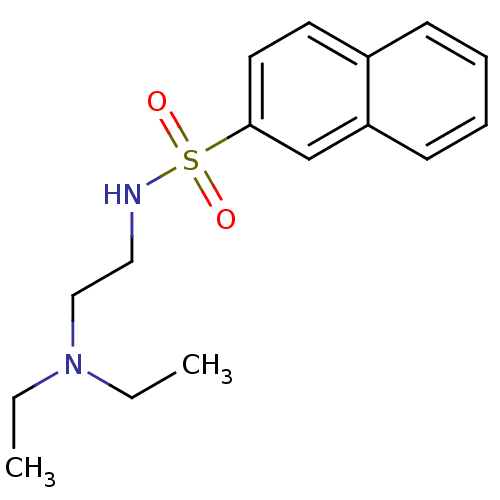
(CHEMBL3087802)Show InChI InChI=1S/C16H22N2O2S/c1-3-18(4-2)12-11-17-21(19,20)16-10-9-14-7-5-6-8-15(14)13-16/h5-10,13,17H,3-4,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443030

(CHEMBL3087808)Show InChI InChI=1S/C13H21ClN2O2S/c1-3-16(4-2)10-9-15-19(17,18)11-12-5-7-13(14)8-6-12/h5-8,15H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE by isothermal titration calorimetry |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443029

(CHEMBL3087809)Show InChI InChI=1S/C14H21F3N2O2S/c1-3-19(4-2)10-9-18-22(20,21)11-12-5-7-13(8-6-12)14(15,16)17/h5-8,18H,3-4,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE by isothermal titration calorimetry |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443028

(CHEMBL3087815)Show InChI InChI=1S/C13H19F3N2O2S/c1-3-18(4-2)9-8-17-21(19,20)12-7-5-6-11(10-12)13(14,15)16/h5-7,10,17H,3-4,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE by isothermal titration calorimetry |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50443027

(CHEMBL3087794)Show InChI InChI=1S/C12H19ClN2O2S/c1-3-15(4-2)9-8-14-18(16,17)12-7-5-6-11(13)10-12/h5-7,10,14H,3-4,8-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.44E+4 | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Binding affinity to mouse AChE by isothermal titration calorimetry |
J Med Chem 56: 7615-24 (2013)
Article DOI: 10.1021/jm400990p
BindingDB Entry DOI: 10.7270/Q24M95Z3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data