

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443692 (CHEMBL3093837) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Linewea... | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443696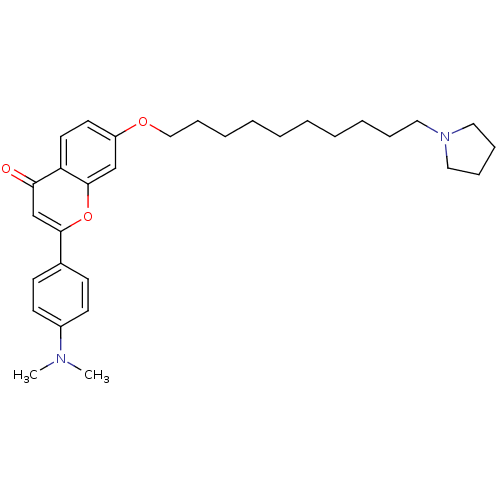 (CHEMBL3093842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443692 (CHEMBL3093837) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443700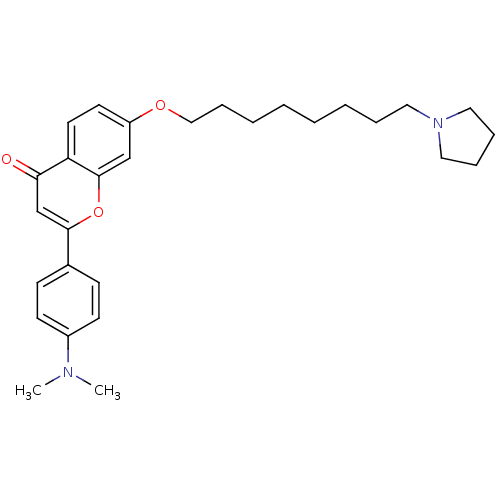 (CHEMBL3093838) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443709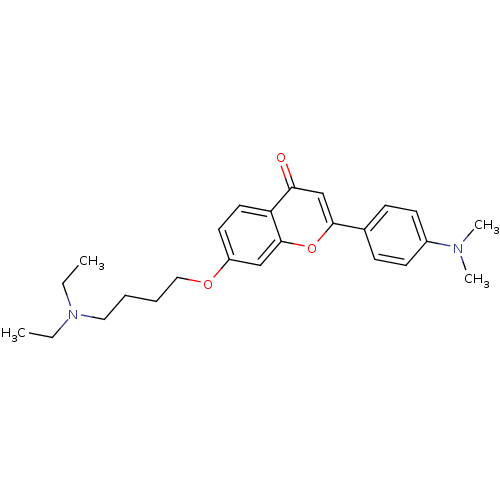 (CHEMBL3093846) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443696 (CHEMBL3093842) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443697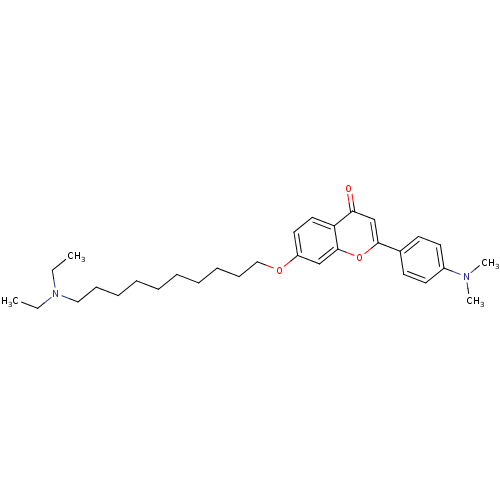 (CHEMBL3093841) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443695 (CHEMBL3093843) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443700 (CHEMBL3093838) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443708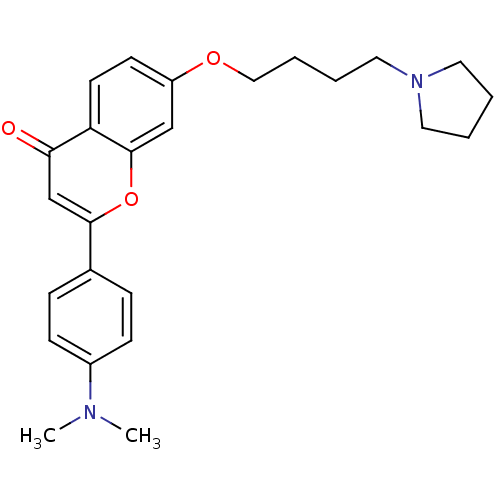 (CHEMBL3093847) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443707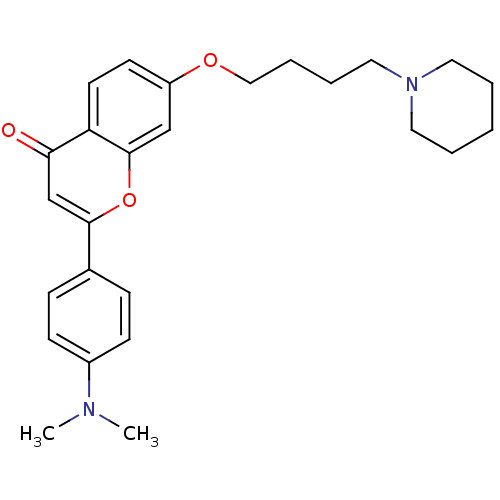 (CHEMBL3093830) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443704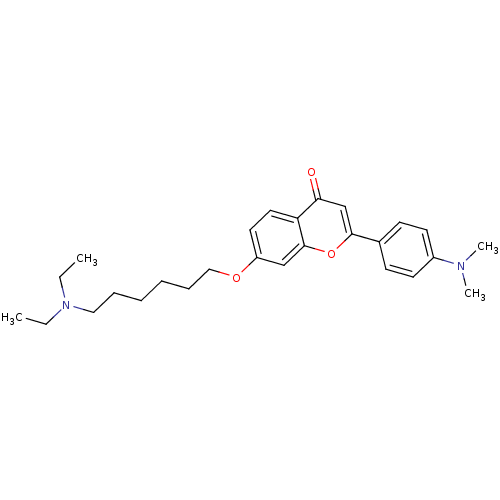 (CHEMBL3093833) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443697 (CHEMBL3093841) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443704 (CHEMBL3093833) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443692 (CHEMBL3093837) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443702 (CHEMBL3093835) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443707 (CHEMBL3093830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443708 (CHEMBL3093847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443702 (CHEMBL3093835) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443701 (CHEMBL3093836) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443709 (CHEMBL3093846) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443699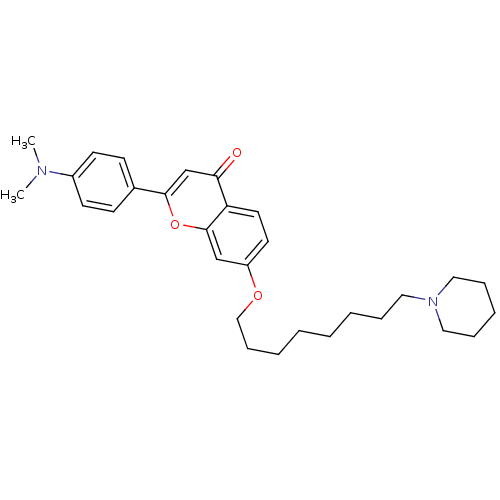 (CHEMBL3093839) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443705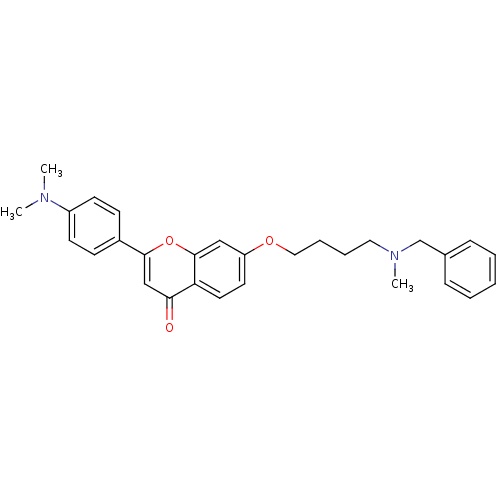 (CHEMBL3093832) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443699 (CHEMBL3093839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443703 (CHEMBL3093834) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443706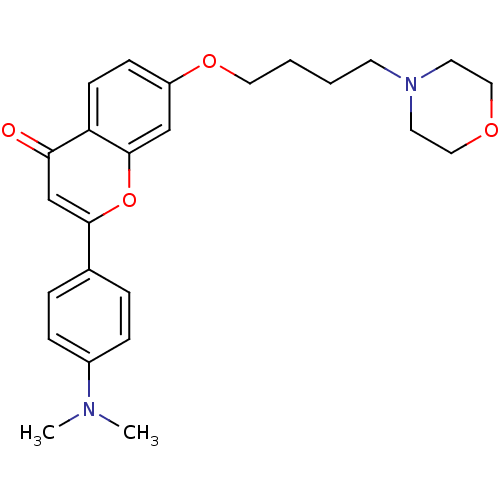 (CHEMBL3093831) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443694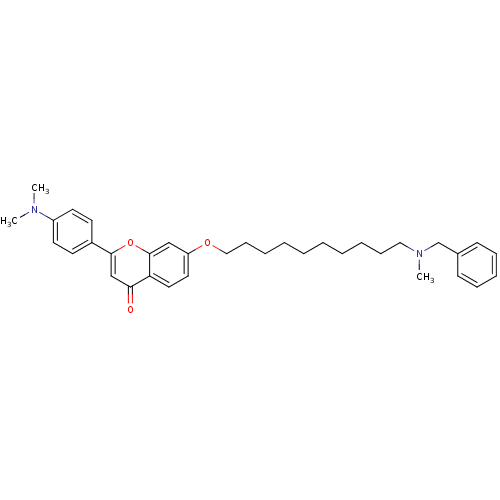 (CHEMBL3093844) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443703 (CHEMBL3093834) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443698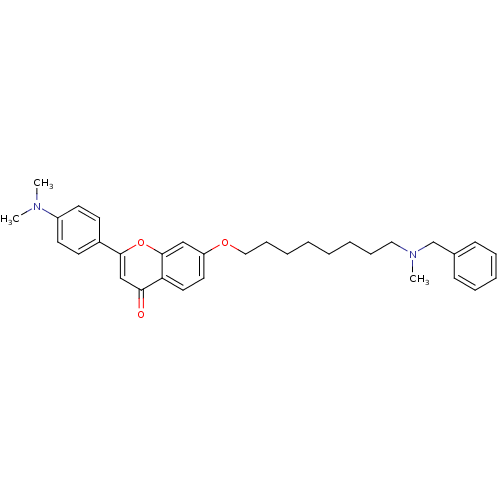 (CHEMBL3093840) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443693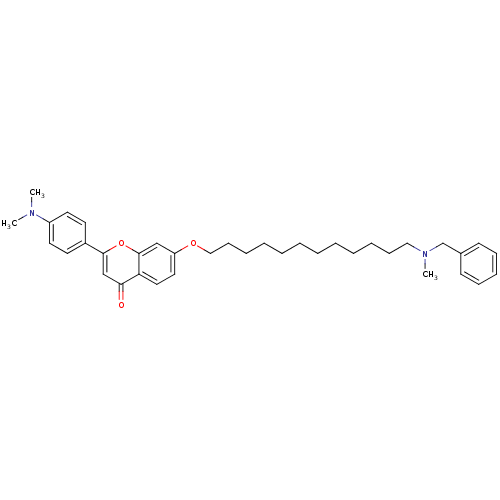 (CHEMBL3093845) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443694 (CHEMBL3093844) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443706 (CHEMBL3093831) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443701 (CHEMBL3093836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443693 (CHEMBL3093845) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443695 (CHEMBL3093843) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443698 (CHEMBL3093840) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50443705 (CHEMBL3093832) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Henan University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butylthiocholine chloride as substrate incubated for 15 mins prior to substrate addition by Ellman's method | Bioorg Med Chem 21: 7275-82 (2013) Article DOI: 10.1016/j.bmc.2013.09.061 BindingDB Entry DOI: 10.7270/Q25B03ZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||