

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443842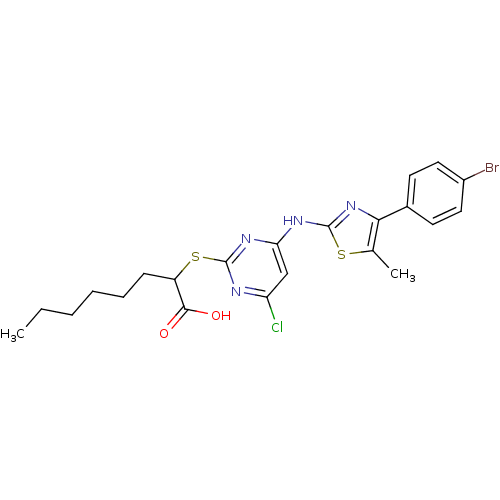 (CHEMBL3094426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443828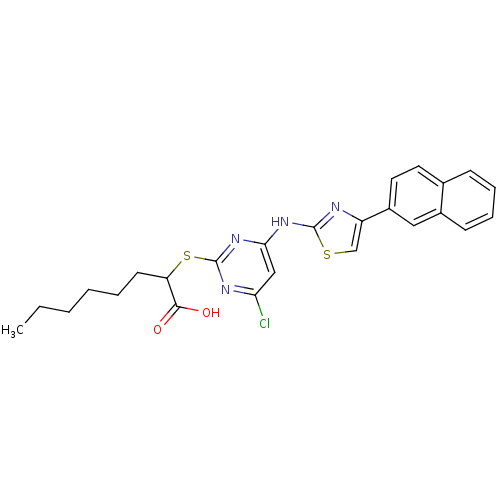 (CHEMBL3094412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443831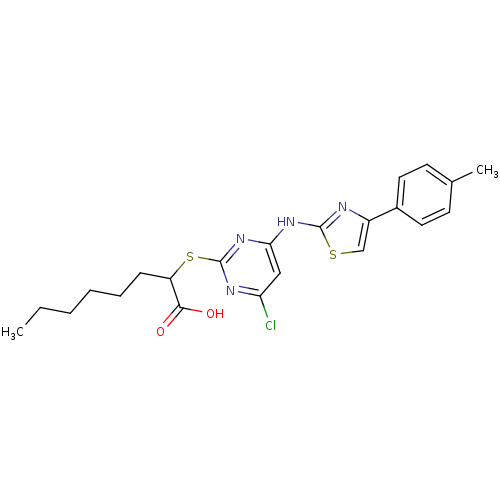 (CHEMBL3091499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443839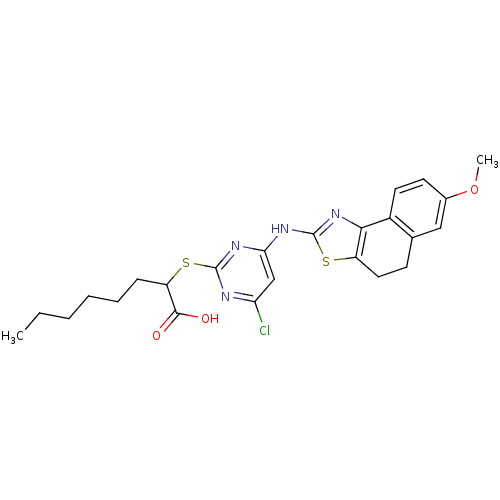 (CHEMBL3094410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443818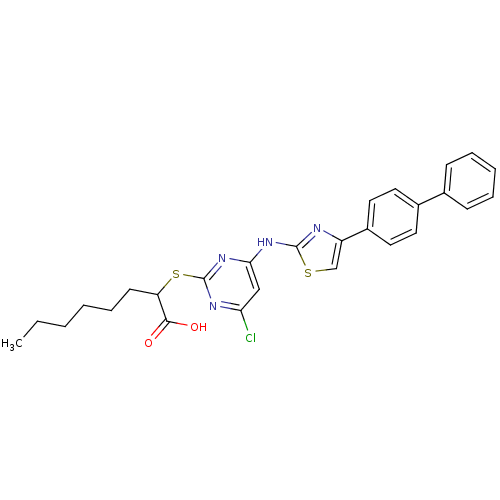 (CHEMBL3094422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443826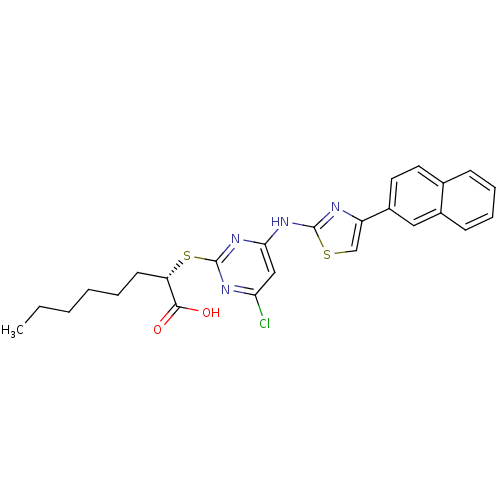 (CHEMBL3094414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443821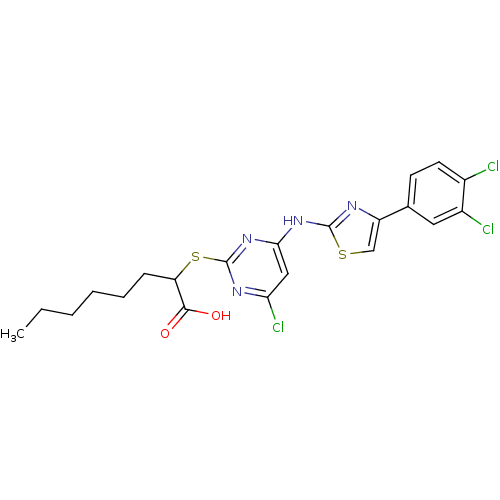 (CHEMBL3094419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443828 (CHEMBL3094412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443830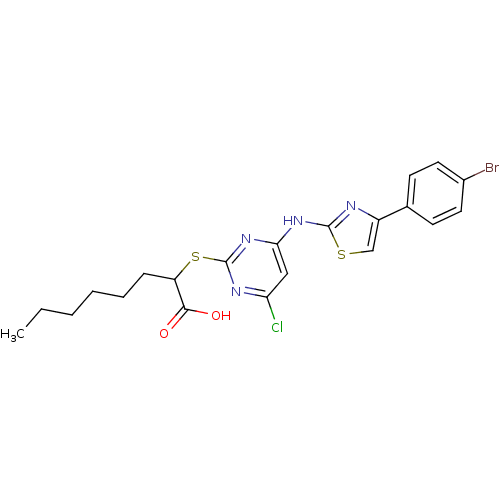 (CHEMBL3091500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443827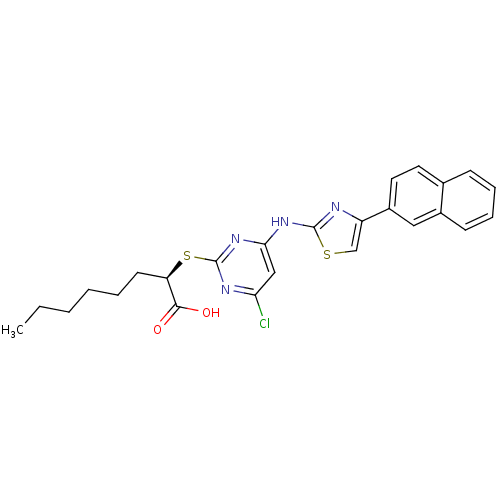 (CHEMBL3094413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443828 (CHEMBL3094412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443822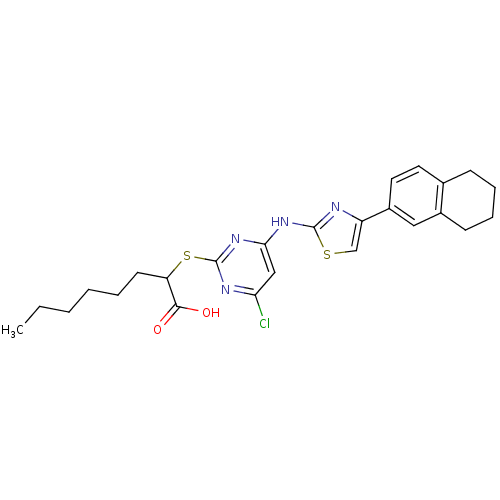 (CHEMBL3094418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443820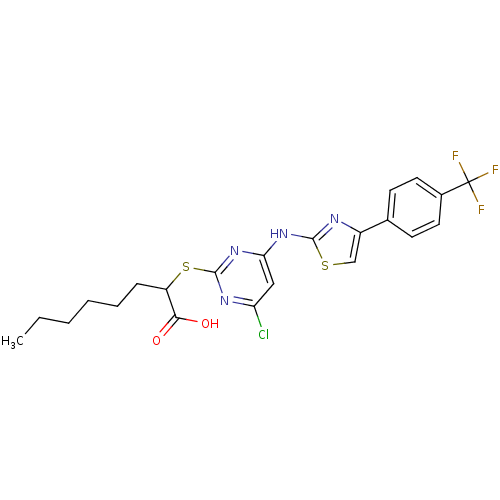 (CHEMBL3094420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443819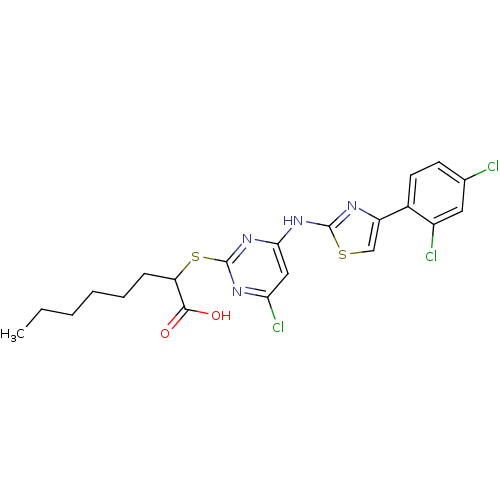 (CHEMBL3094421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443833 (CHEMBL3091497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443829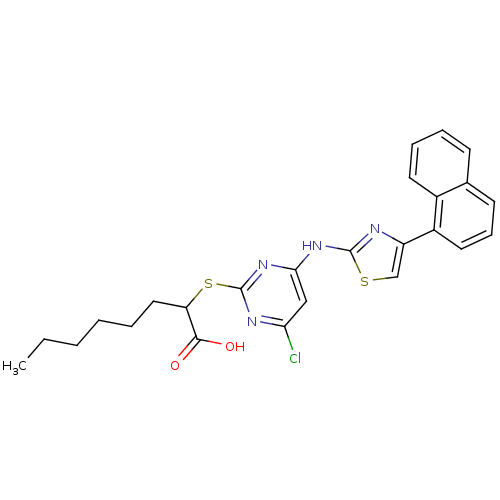 (CHEMBL3094411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443827 (CHEMBL3094413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443822 (CHEMBL3094418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443821 (CHEMBL3094419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443833 (CHEMBL3091497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443818 (CHEMBL3094422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443832 (CHEMBL3091498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443827 (CHEMBL3094413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443815 (CHEMBL3094425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443818 (CHEMBL3094422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443840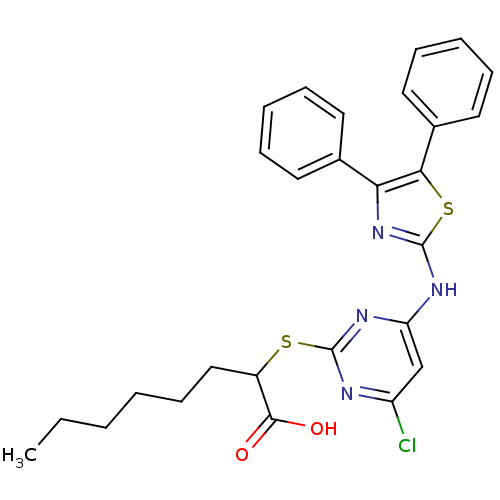 (CHEMBL3094428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443826 (CHEMBL3094414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443840 (CHEMBL3094428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443820 (CHEMBL3094420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443843 (CHEMBL3094429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443815 (CHEMBL3094425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443831 (CHEMBL3091499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443838 (CHEMBL3094431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443840 (CHEMBL3094428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443821 (CHEMBL3094419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443829 (CHEMBL3094411) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443830 (CHEMBL3091500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443833 (CHEMBL3091497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443841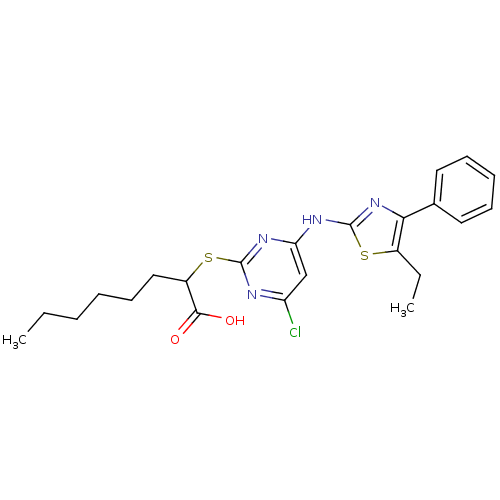 (CHEMBL3094427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50273709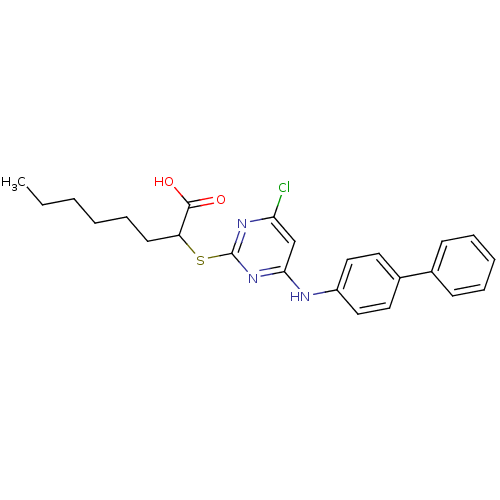 (2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443830 (CHEMBL3091500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443819 (CHEMBL3094421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443838 (CHEMBL3094431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate incubated 5 to 10 mins prior to... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50443832 (CHEMBL3091498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of mPGES-1 from human A549 cell microsomal membranes using PGH2 as substrate incubated 15 mins prior to substrate addition measured after ... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443817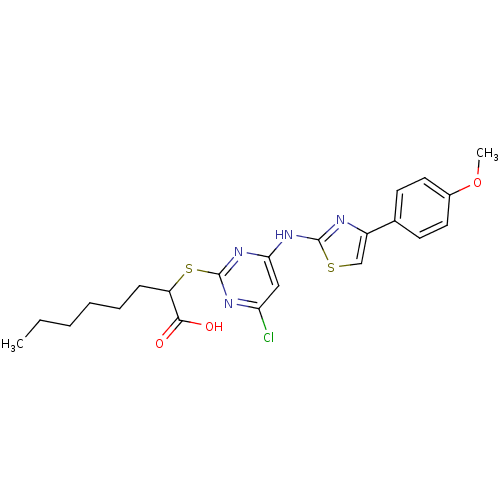 (CHEMBL3094423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443826 (CHEMBL3094414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50443837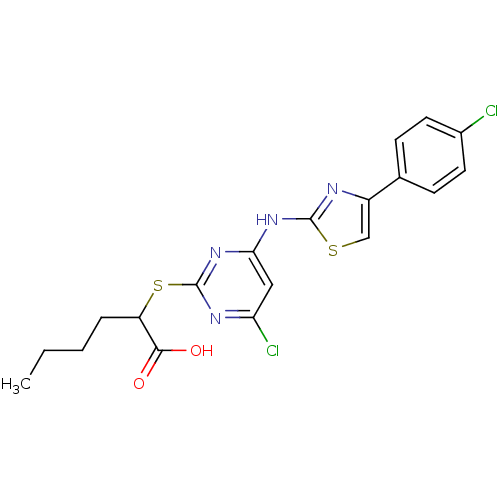 (CHEMBL3094432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human polymorphonuclear leukocytes using arachidonic acid as substrate incubated 15 mins prior to s... | J Med Chem 56: 9031-44 (2013) Article DOI: 10.1021/jm401557w BindingDB Entry DOI: 10.7270/Q24B32R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 95 total ) | Next | Last >> |