

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444072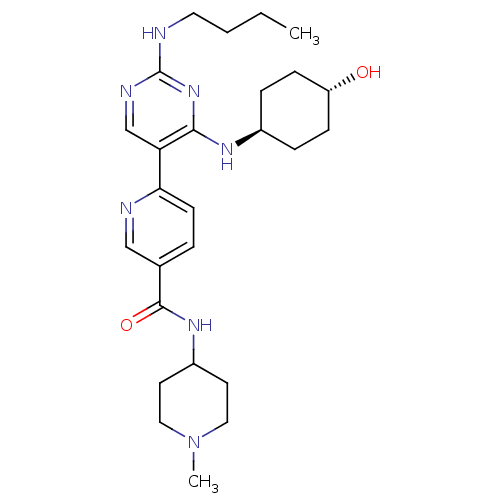 (CHEMBL3092793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444070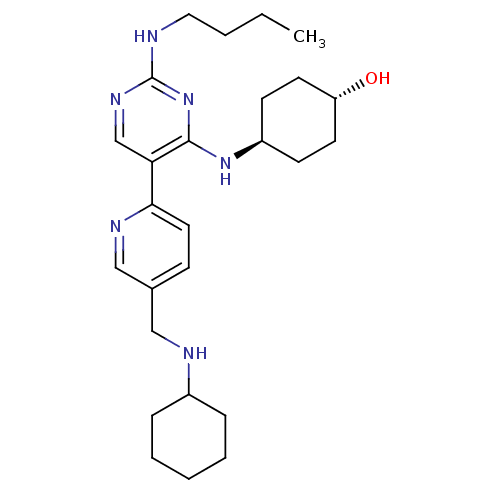 (CHEMBL3092795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444073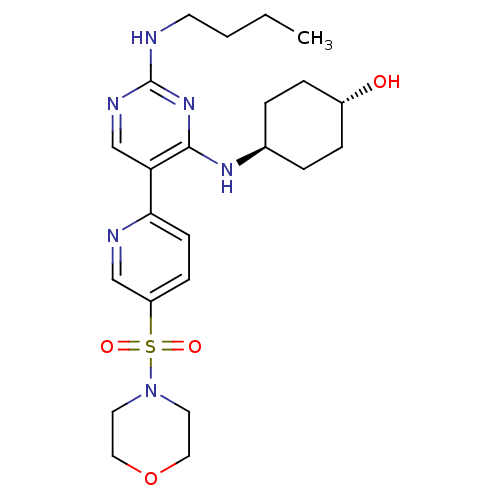 (CHEMBL3092792) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444068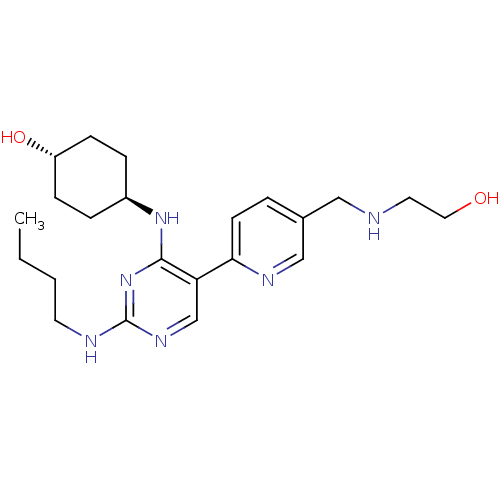 (CHEMBL3092797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444075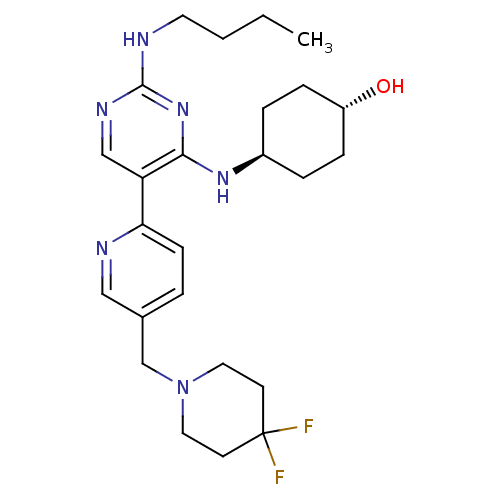 (CHEMBL3092790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444069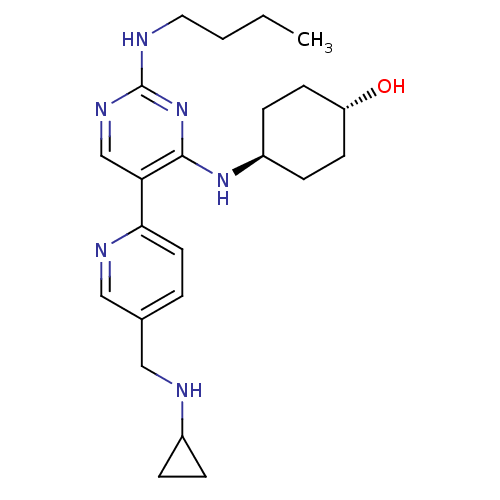 (CHEMBL3092796) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444042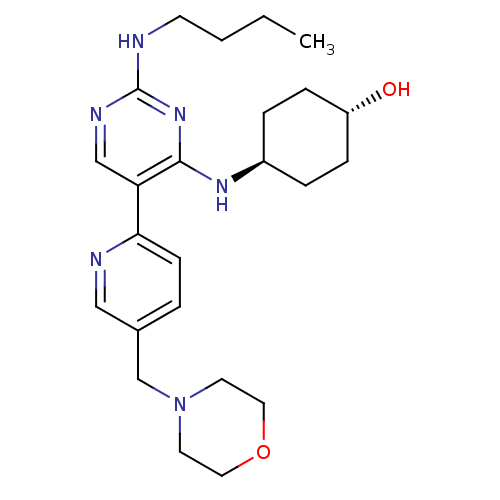 (CHEMBL3092807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444074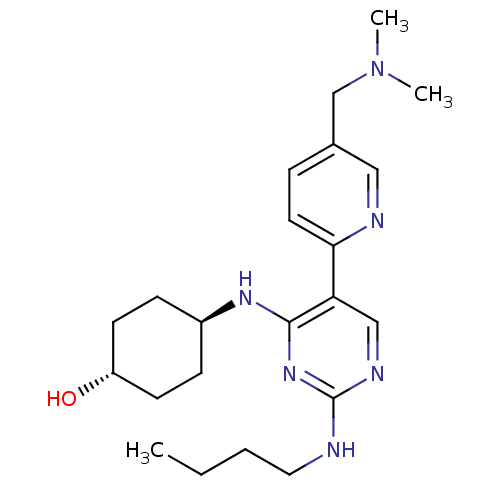 (CHEMBL3092791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444066 (CHEMBL3092799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444080 (CHEMBL3092805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444071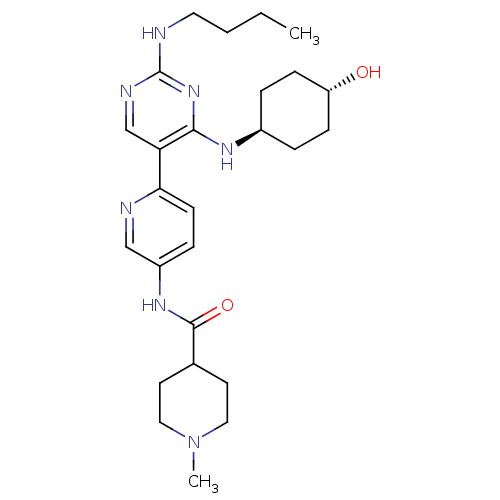 (CHEMBL3092794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444079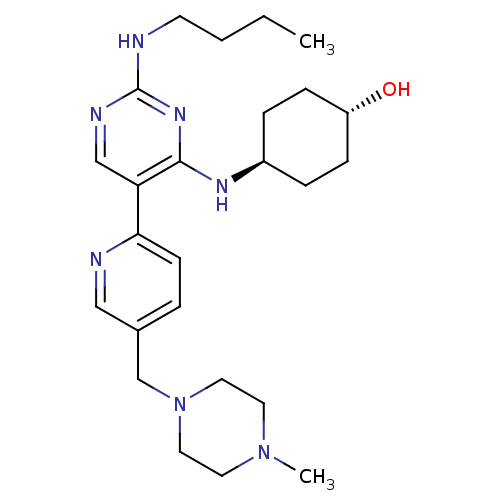 (CHEMBL3092806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444065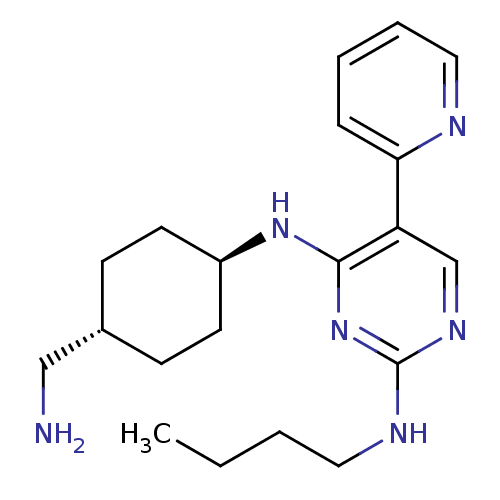 (CHEMBL3092800) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444081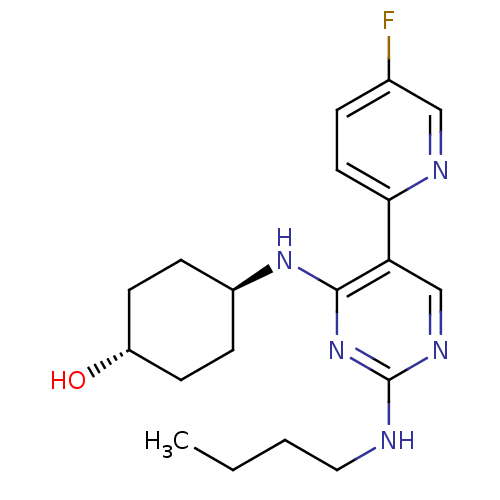 (CHEMBL3092804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444042 (CHEMBL3092807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase phosphorylation in human 697 B-ALL cells after 1 hr by Western blot analysis | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444077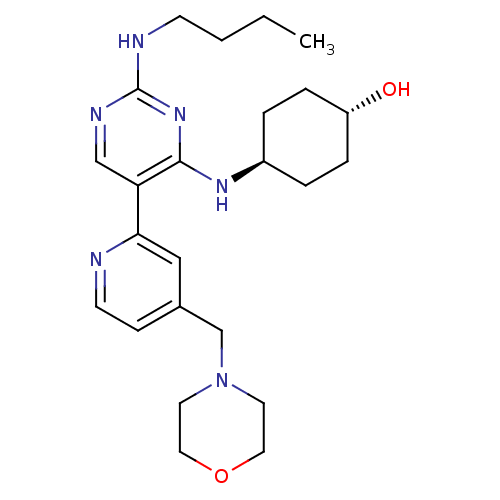 (CHEMBL3092788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444046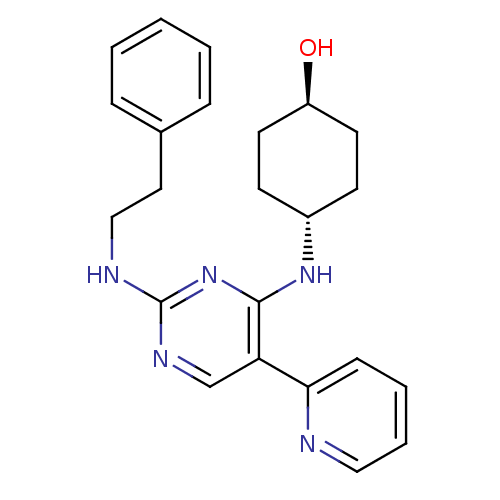 (CHEMBL3092781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444085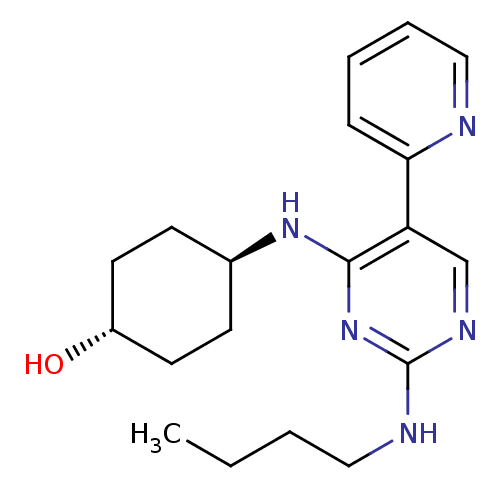 (CHEMBL3092785 | US9649309, Compound UNC1917A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444041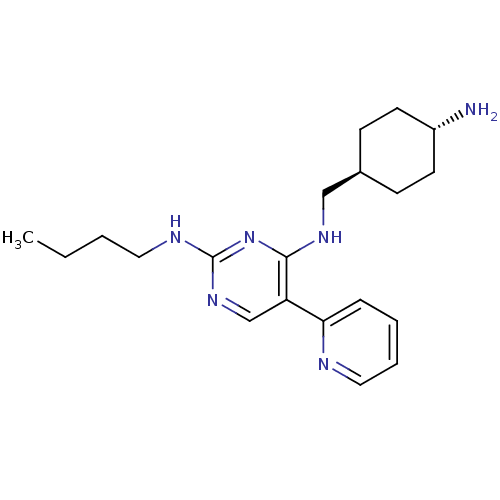 (CHEMBL3092787) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444064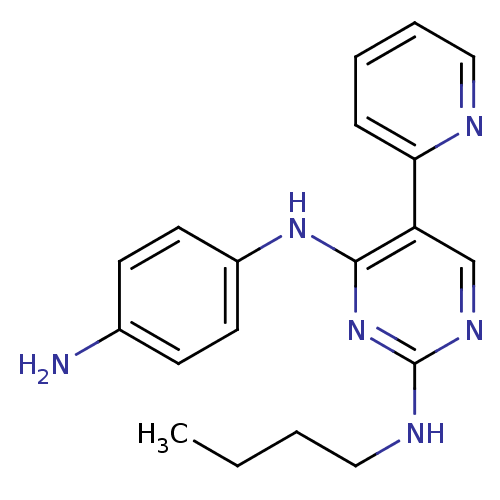 (CHEMBL3092801 | US9649309, Compound UNC2999A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444076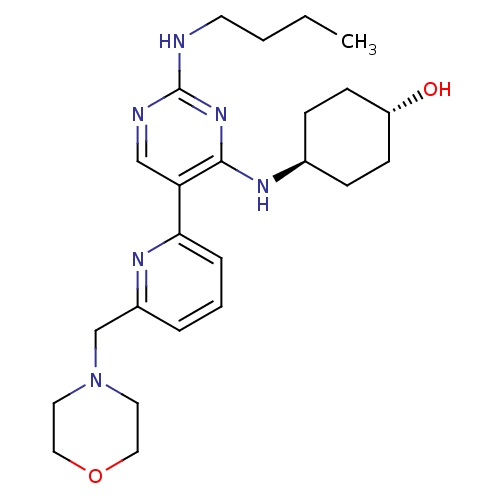 (CHEMBL3092789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444052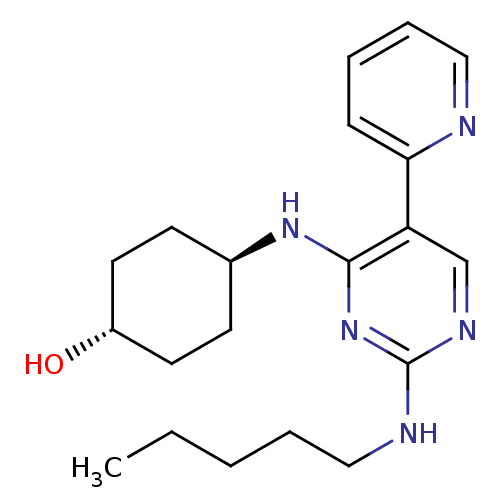 (CHEMBL3092775 | US9649309, Compound UNC3022A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444074 (CHEMBL3092791) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444068 (CHEMBL3092797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444072 (CHEMBL3092793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444070 (CHEMBL3092795) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444073 (CHEMBL3092792) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444069 (CHEMBL3092796) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444063 (CHEMBL3092809 | US9649309, Compound UNC2961A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444075 (CHEMBL3092790) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444070 (CHEMBL3092795) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444051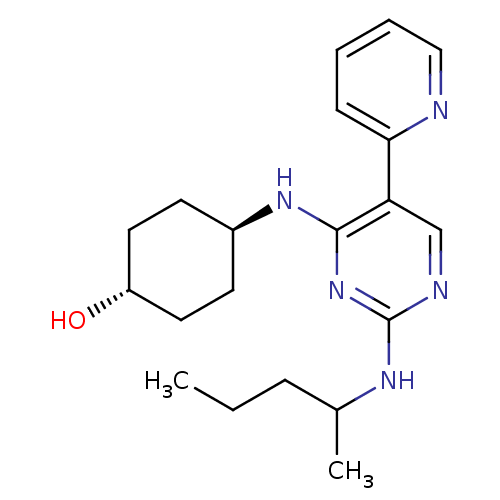 (CHEMBL3092776 | US9649309, Compound UNC3054A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444043 (CHEMBL3092784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444053 (CHEMBL3092774 | US9649309, Compound UNC3020A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444065 (CHEMBL3092800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444072 (CHEMBL3092793) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444074 (CHEMBL3092791) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444068 (CHEMBL3092797) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444073 (CHEMBL3092792) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444062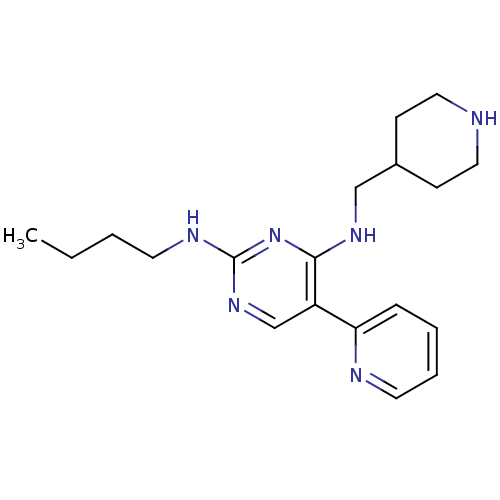 (CHEMBL3092765 | US9649309, Compound UNC2962A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444046 (CHEMBL3092781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444048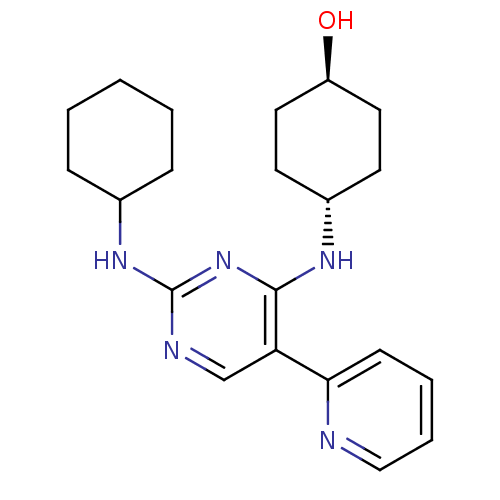 (CHEMBL3092779 | US9649309, Compound UNC3053A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444069 (CHEMBL3092796) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444066 (CHEMBL3092799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444080 (CHEMBL3092805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444071 (CHEMBL3092794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444042 (CHEMBL3092807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50444079 (CHEMBL3092806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Tyro-3 kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis as... | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444047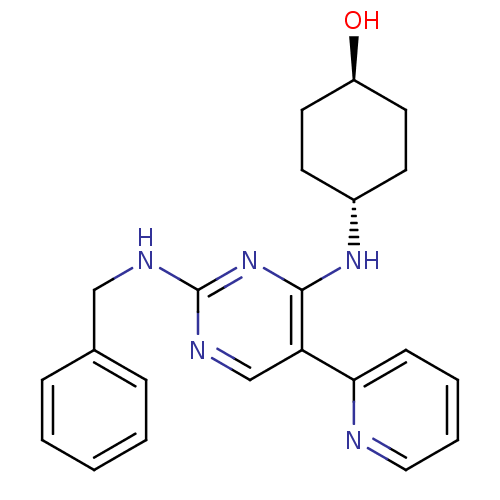 (CHEMBL3092780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50444075 (CHEMBL3092790) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Axl kinase (unknown origin) using 5-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 135 total ) | Next | Last >> |