

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446341 (CHEMBL3109581) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446342 (CHEMBL3109579) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50261110 (CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446339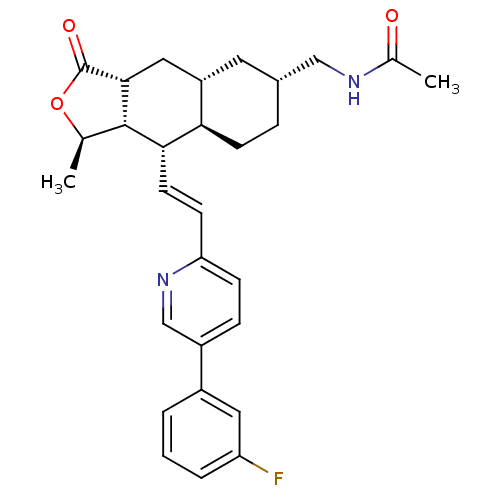 (CHEMBL3109583) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446332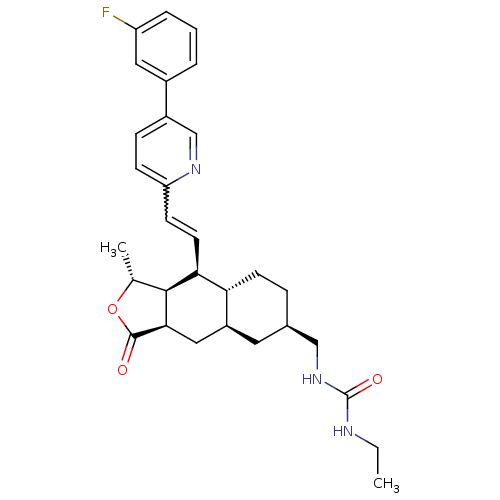 (CHEMBL3109590) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446338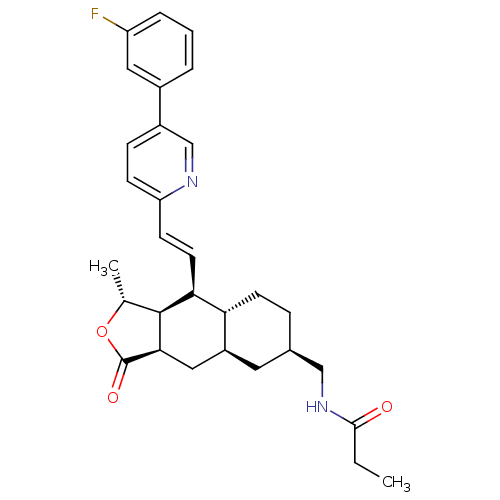 (CHEMBL3109584) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446335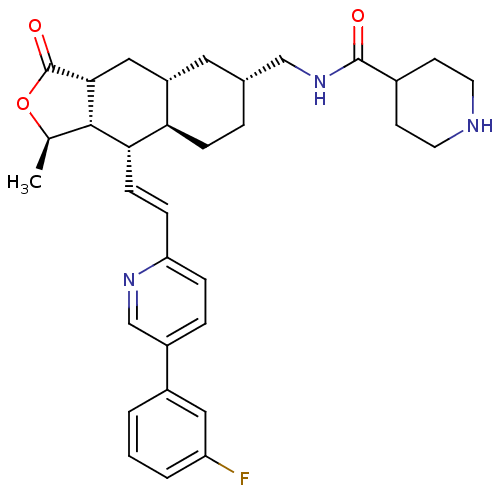 (CHEMBL3109587) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446340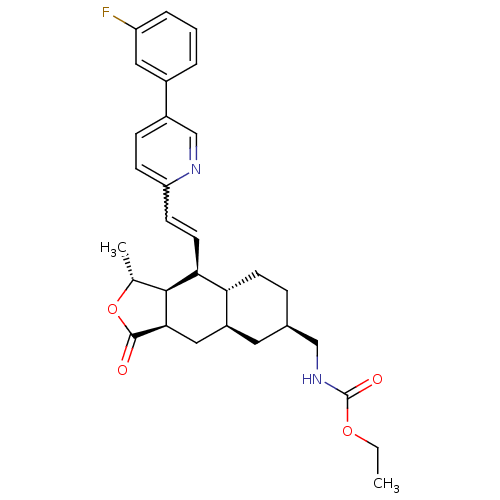 (CHEMBL3109582) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446334 (CHEMBL3109588) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446333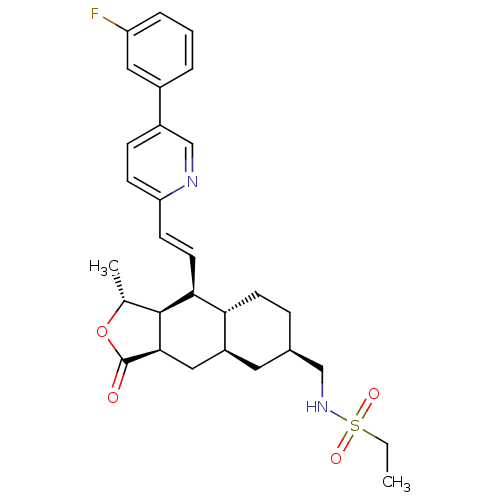 (CHEMBL3109589) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446343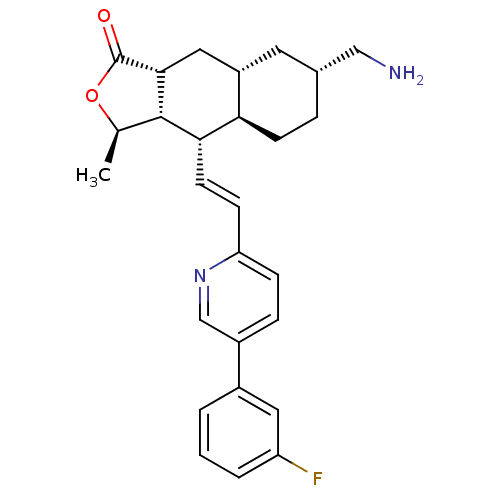 (CHEMBL3109580) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446344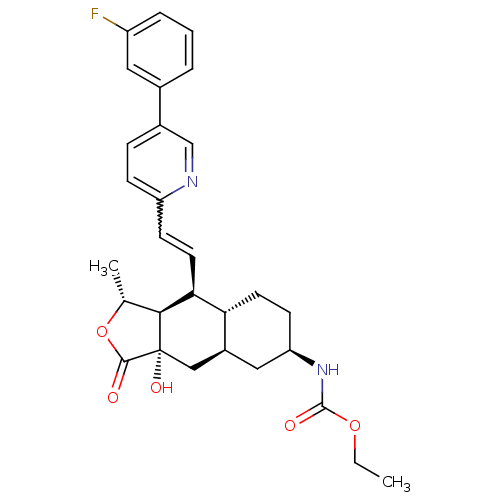 (CHEMBL3109578) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446336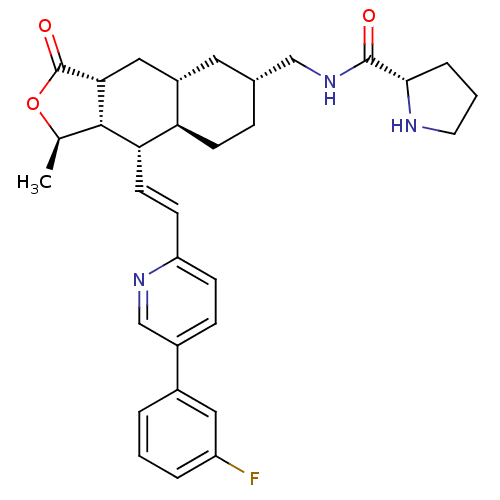 (CHEMBL3109586) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446331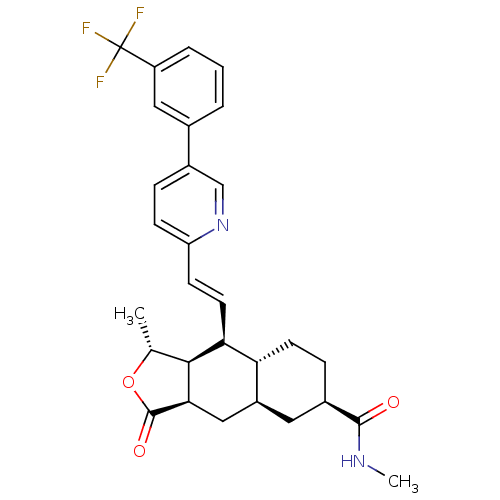 (CHEMBL3109591) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446329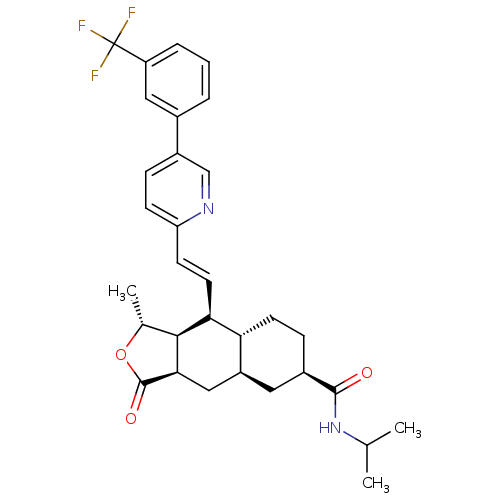 (CHEMBL3109572) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446337 (CHEMBL3109585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446330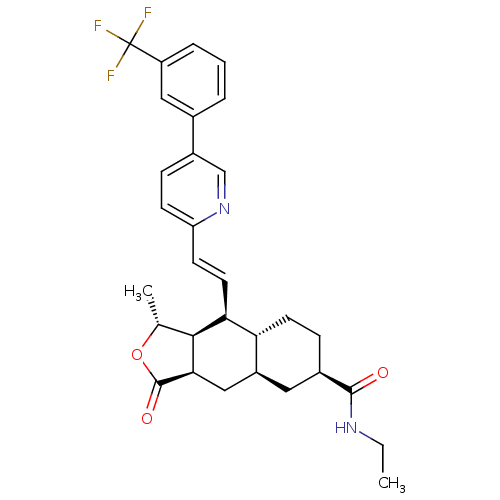 (CHEMBL3109571) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446328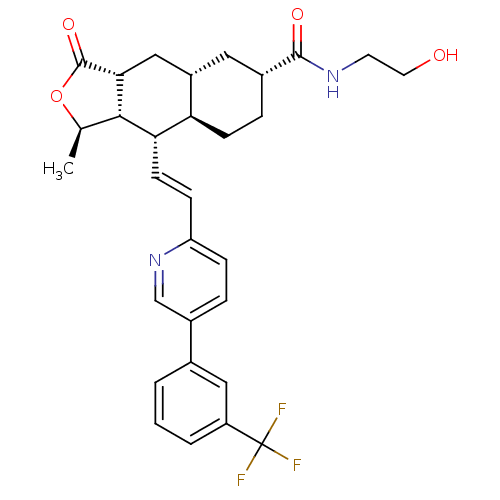 (CHEMBL3109573) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446327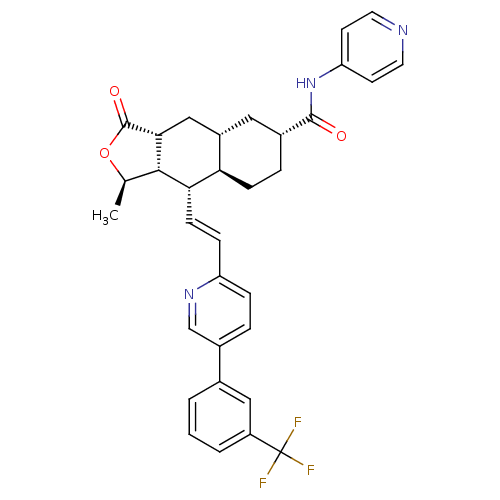 (CHEMBL3109574) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446325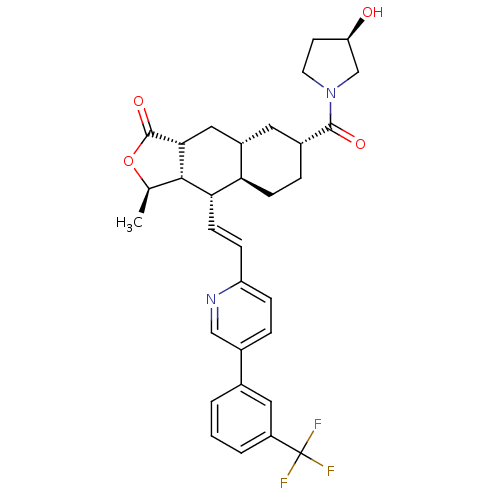 (CHEMBL3109576) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446326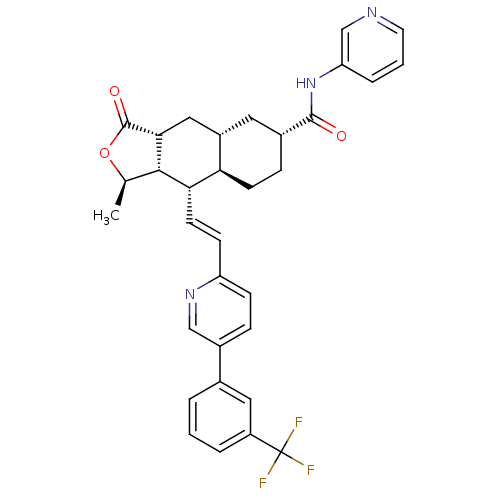 (CHEMBL3109575) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50446324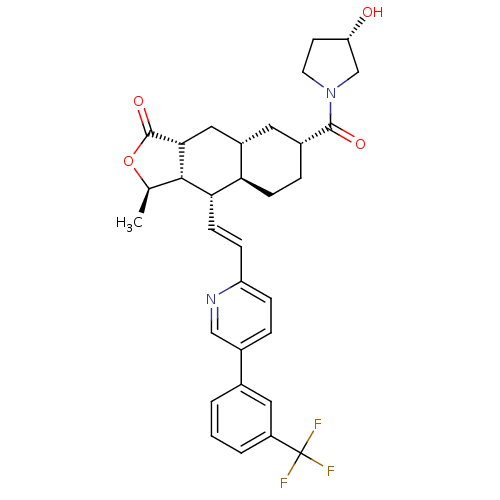 (CHEMBL3109577) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]haTRAP from PAR-1 isolated from human platelets by liquid scintillation counting analysis | ACS Med Chem Lett 5: 183-7 (2014) Article DOI: 10.1021/ml400452v BindingDB Entry DOI: 10.7270/Q2V40WPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||