

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30132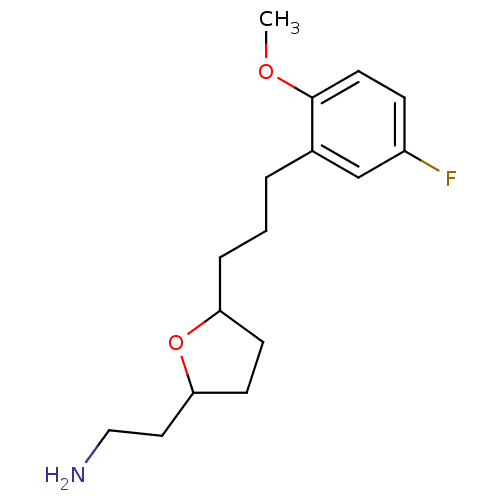 (CHEMBL450907 | tetrahydrofuranyl ethylamine, 15) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -50.6 | n/a | n/a | 2.30 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.10 | -50.6 | n/a | n/a | 7.30 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM30129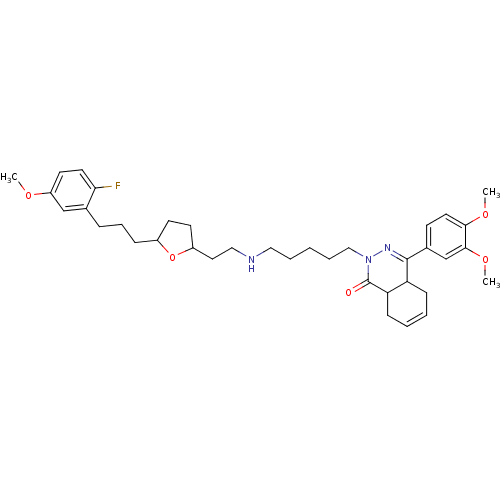 (phthalazinone, 22) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | -52.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM30128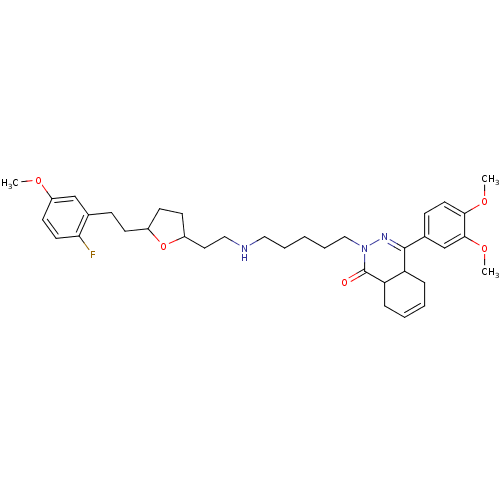 (phthalazinone, 21) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30131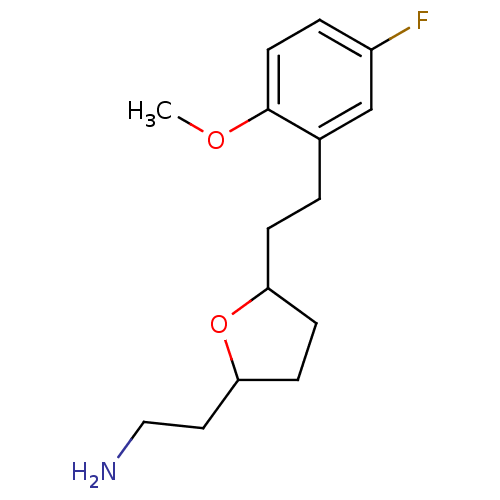 (tetrahydrofuranyl ethylamine, 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | 2.5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM30127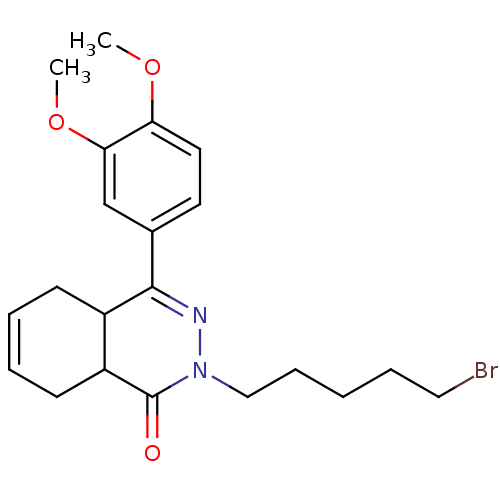 (CHEMBL480988 | phthalazinone, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | -50.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM30126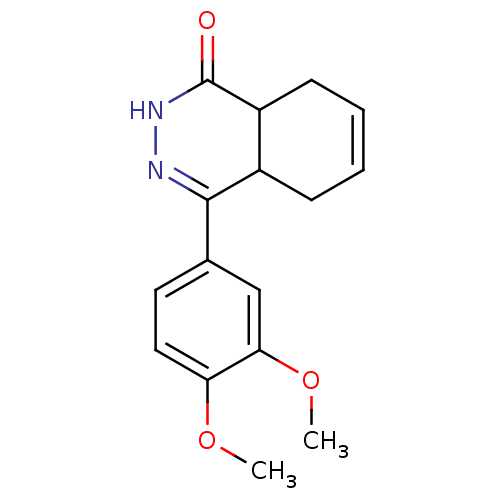 (CHEMBL313041 | phthalazinone, 19) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | -48.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30133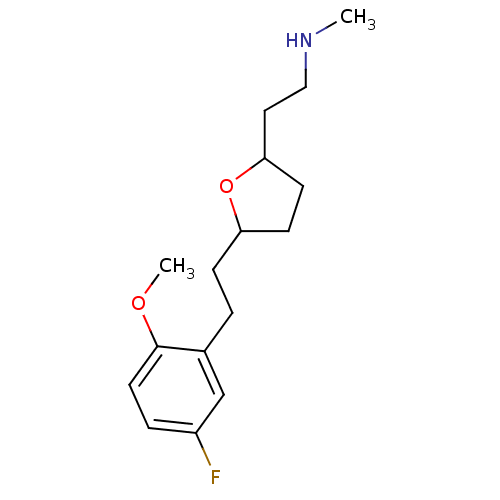 (tetrahydrofuranyl ethylamine, 24) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 21.2 | -43.4 | n/a | n/a | 18.4 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 58.9 | -42.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30128 (phthalazinone, 21) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 156 | -38.5 | n/a | n/a | 127 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30129 (phthalazinone, 22) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 194 | -37.9 | n/a | n/a | 194 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM30128 (phthalazinone, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 199 | -39.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM30126 (CHEMBL313041 | phthalazinone, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | -38.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22418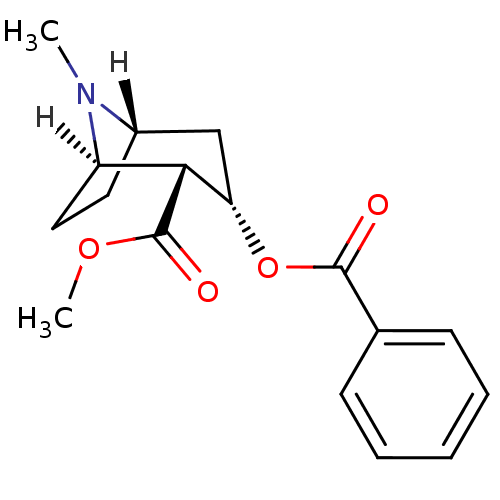 (Cocaine | Cocaine (-) | methyl (1R,2R,3S,5S)-3-(be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 276 | -37.1 | n/a | n/a | 416 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22418 (Cocaine | Cocaine (-) | methyl (1R,2R,3S,5S)-3-(be...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 371 | -36.3 | n/a | n/a | 303 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM30129 (phthalazinone, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | -37.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM30129 (phthalazinone, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | >-35.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM30128 (phthalazinone, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | >-35.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM30126 (CHEMBL313041 | phthalazinone, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | >-35.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 34 |
Human BioMolecular Research Institute | Assay Description The samples were assayed in a solution composed of PDE4, [3H]cAMP/cAMP, and test compounds. To convert AMP to adenosine, snake venom was added to eac... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM22418 (Cocaine | Cocaine (-) | methyl (1R,2R,3S,5S)-3-(be...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.12E+3 | n/a | n/a | n/a | 835 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.56E+3 | n/a | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30128 (phthalazinone, 21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | 957 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30131 (tetrahydrofuranyl ethylamine, 14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.44E+3 | n/a | n/a | n/a | 481 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30128 (phthalazinone, 21) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.48E+3 | -31.7 | n/a | n/a | 3.56E+3 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30129 (phthalazinone, 22) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.34E+3 | -29.8 | n/a | n/a | 5.34E+3 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30131 (tetrahydrofuranyl ethylamine, 14) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.50E+3 | -29.3 | n/a | n/a | 2.79E+4 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.67E+3 | -29.2 | n/a | n/a | 1.95E+4 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30132 (CHEMBL450907 | tetrahydrofuranyl ethylamine, 15) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.31E+3 | -29.0 | n/a | n/a | 1.70E+4 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30129 (phthalazinone, 22) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.44E+3 | n/a | n/a | n/a | 7.44E+3 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30132 (CHEMBL450907 | tetrahydrofuranyl ethylamine, 15) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06E+4 | n/a | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30133 (tetrahydrofuranyl ethylamine, 24) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30127 (CHEMBL480988 | phthalazinone, 20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.91E+4 | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30133 (tetrahydrofuranyl ethylamine, 24) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+4 | -23.6 | n/a | n/a | >1.00E+5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM30126 (CHEMBL313041 | phthalazinone, 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30127 (CHEMBL480988 | phthalazinone, 20) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | >1.00E+5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30126 (CHEMBL313041 | phthalazinone, 19) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | >1.00E+5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM30127 (CHEMBL480988 | phthalazinone, 20) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | >1.00E+5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM30126 (CHEMBL313041 | phthalazinone, 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | >1.00E+5 | n/a | n/a | 7.4 | 22 |
Human BioMolecular Research Institute | Assay Description Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ... | J Med Chem 52: 1530-9 (2009) Article DOI: 10.1021/jm8010993 BindingDB Entry DOI: 10.7270/Q2MK6B7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||