

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297395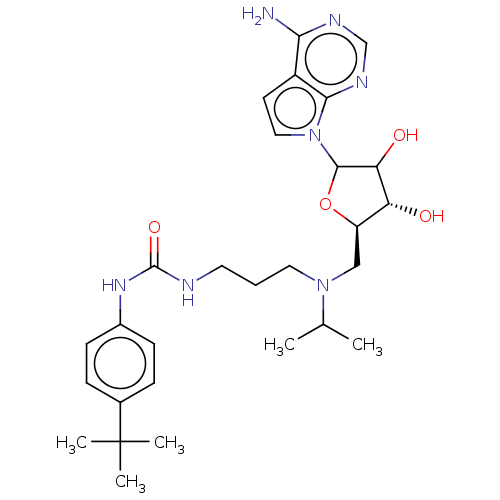 (US10112968, Compound D16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | 0.0100 | n/a | 0.000300 | 3.00E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297394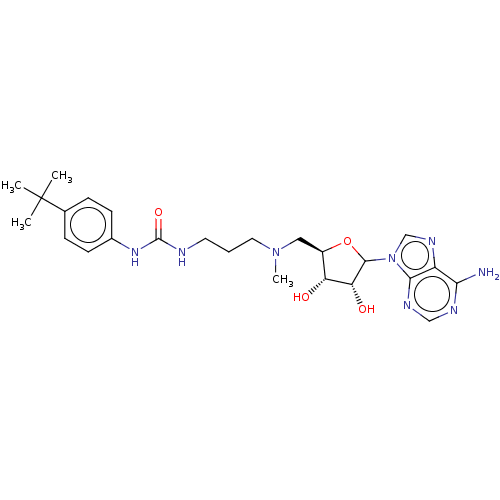 (US10112968, Compound C118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | 1.70 | n/a | 0.0200 | 1.20E+7 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297392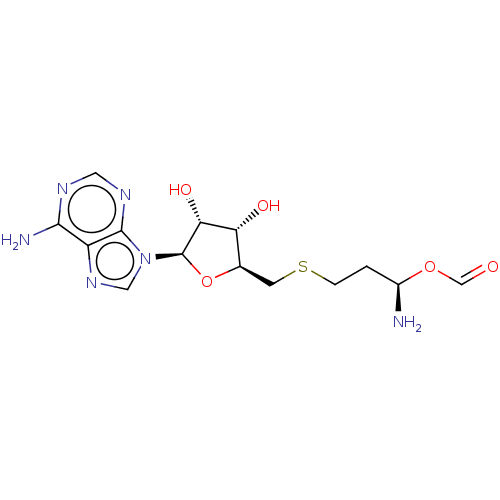 (US10112968, Compound SAH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 320 | n/a | n/a | 71 | n/a | 0.100 | 1.40E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297393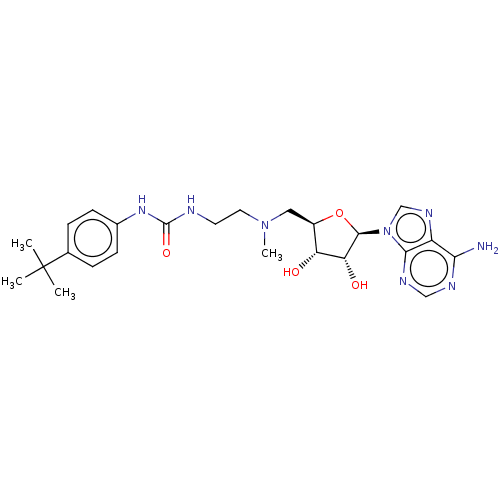 (US10112968, Compound C94) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 845 | n/a | n/a | 167 | n/a | 0.200 | 1.20E+6 | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297391 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297390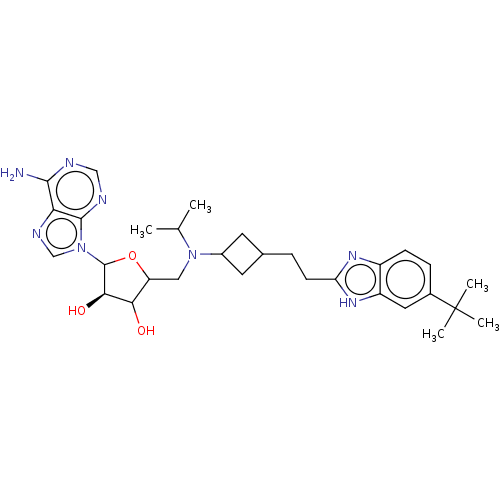 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1s,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM297389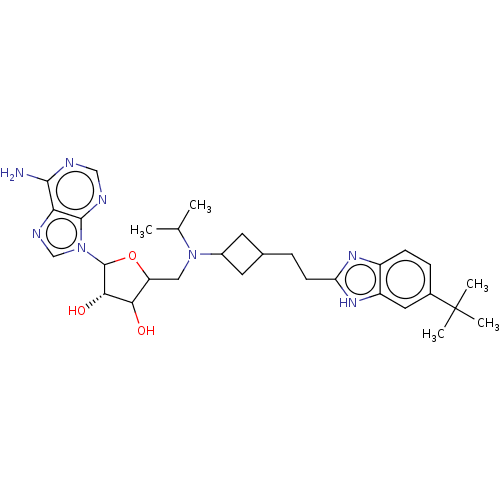 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description Unless otherwise indicated, assays of DOT1L enzymatic activity were performed under balanced conditions (all substrates present at concentrations equ... | US Patent US10112968 (2018) BindingDB Entry DOI: 10.7270/Q20R9RFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||