

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33410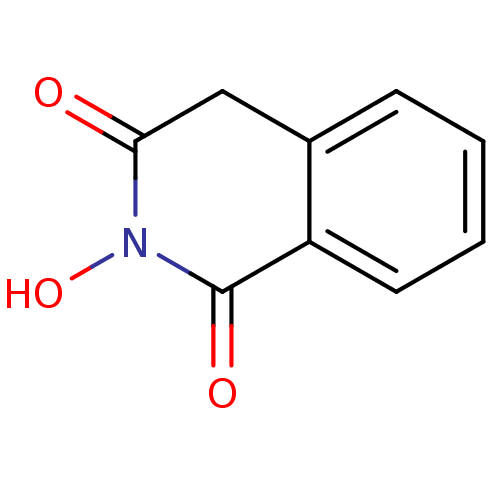 (CHEMBL16755 | N-hydroxyisoquinolinedione, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33419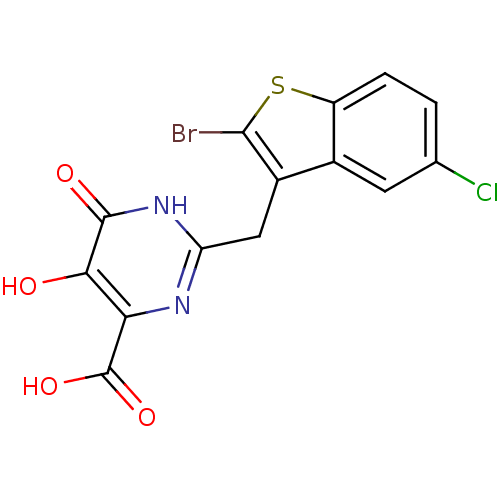 (pyrimidinol carboxylic acid, 11) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33411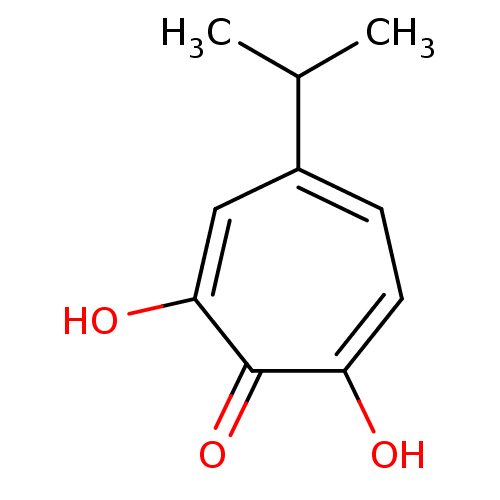 (β-Thujaplicinol | hydroxytropolone, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33418 (pyrimidinol carboxylic acid, 10) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33417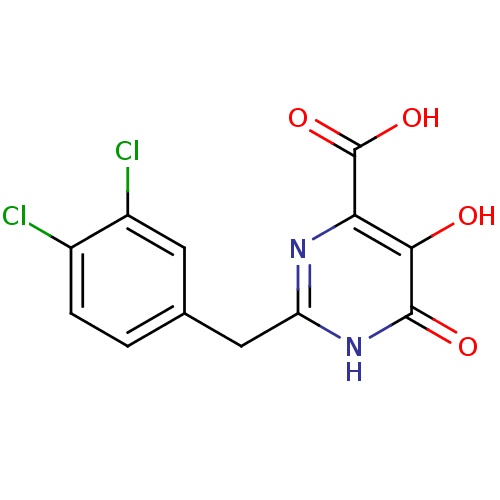 (pyrimidinol carboxylic acid, 9) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33415 (CHEMBL204900 | pyrimidinol carboxylic acid, 7) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33412 (CHEMBL183852 | N-hydroxy quinazolinedione, 4 | US1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33409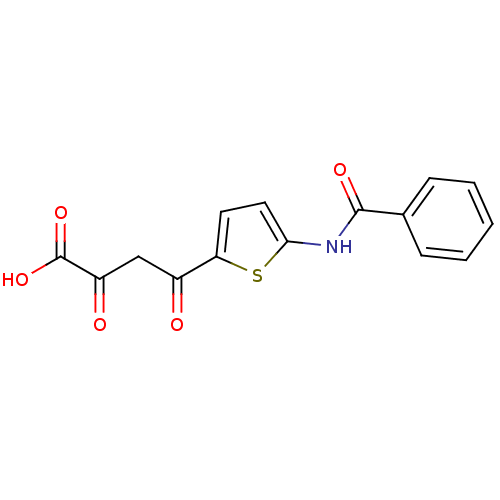 (alpha, gamma-diketo acid, 1) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.85E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33416 (pyrimidinol carboxylic acid, 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33414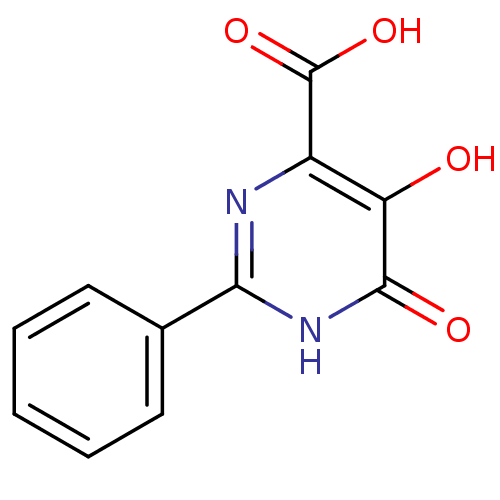 (CHEMBL440562 | pyrimidinol carboxylic acid, 6) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33413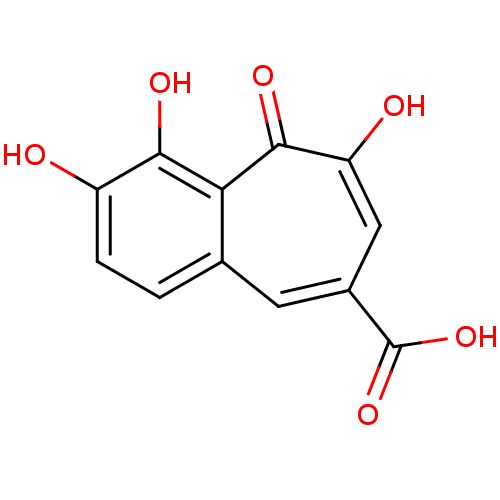 (benzo[7]annulene-8-carboxylic acid, 5) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33419 (pyrimidinol carboxylic acid, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.85E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM33420 (pyrimidinol carboxylic acid, 12) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33418 (pyrimidinol carboxylic acid, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33415 (CHEMBL204900 | pyrimidinol carboxylic acid, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33416 (pyrimidinol carboxylic acid, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33417 (pyrimidinol carboxylic acid, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease H1 (Homo sapiens (Human)) | BDBM33414 (CHEMBL440562 | pyrimidinol carboxylic acid, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 20 |
Gilead Sciences Inc. | Assay Description Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol... | J Med Chem 52: 5781-4 (2009) Article DOI: 10.1021/jm900597q BindingDB Entry DOI: 10.7270/Q2TM78F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||