

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384541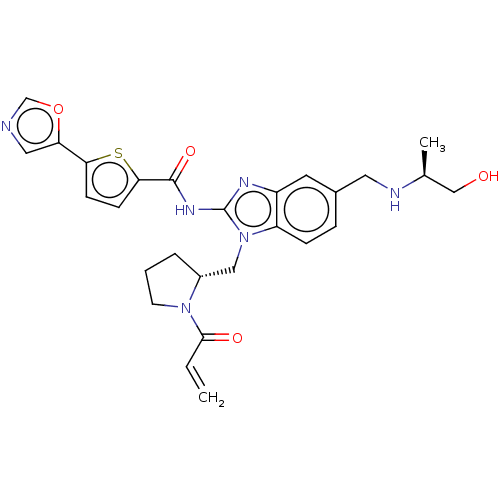 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384539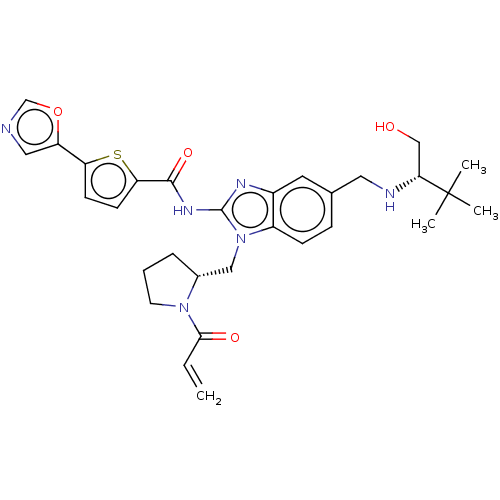 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384538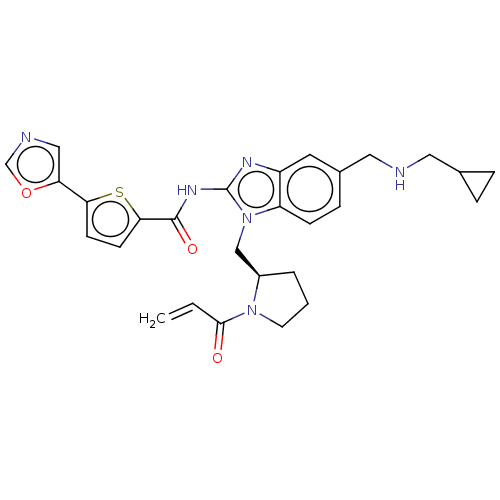 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384543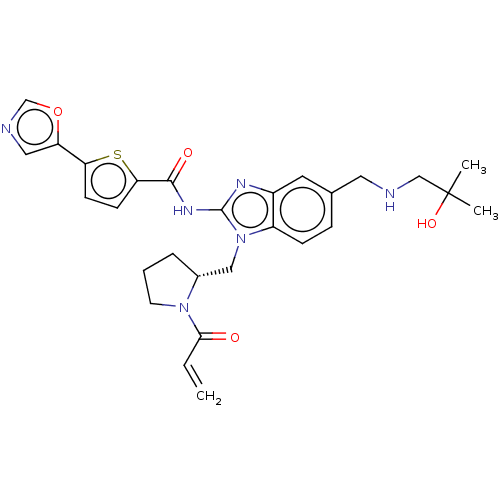 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384542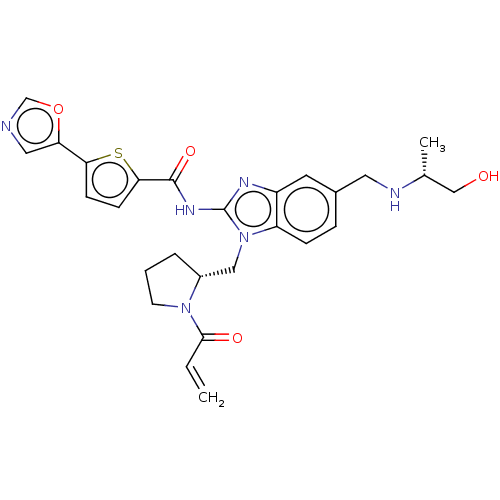 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384540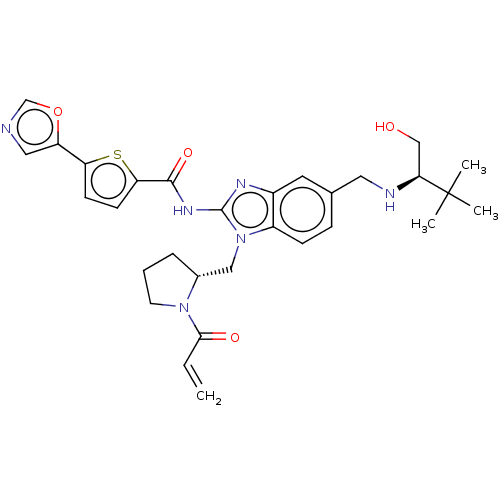 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384539 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384542 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384544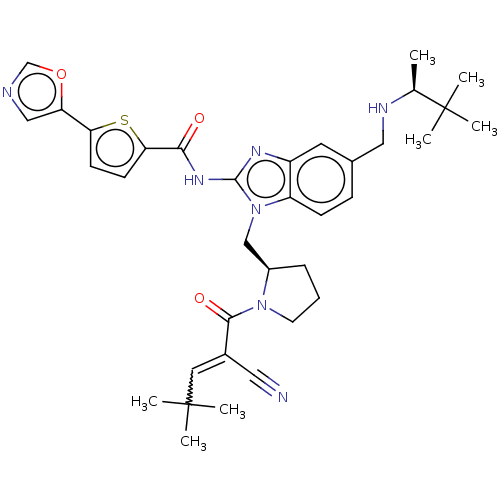 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384538 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384541 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384543 ((R)-N-(1-((1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384540 (N-(1-(((R)-1-acryloylpyrrolidin-2-yl)methyl)-5-(((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384534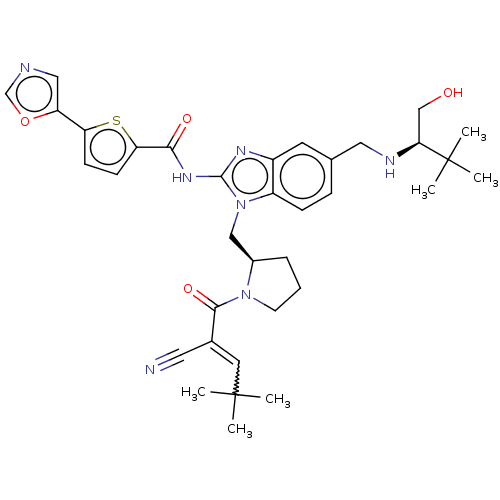 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384535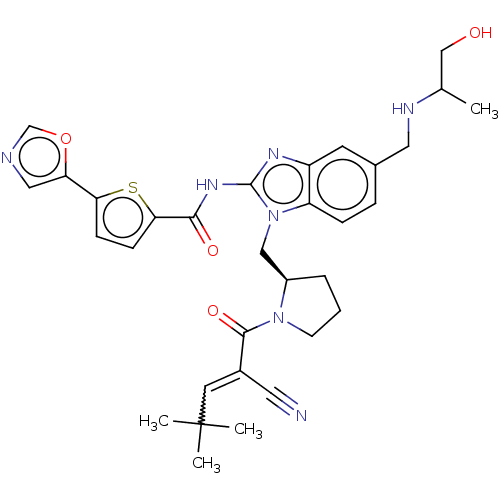 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384533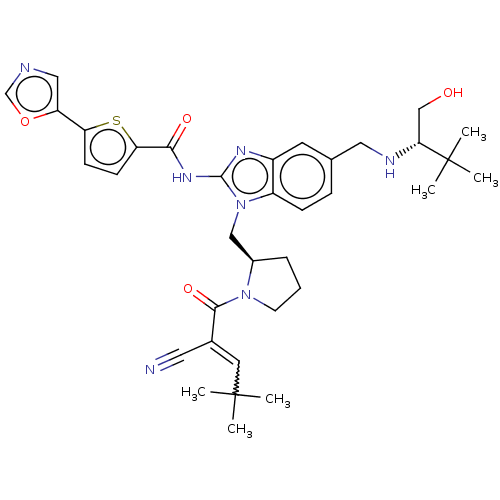 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384537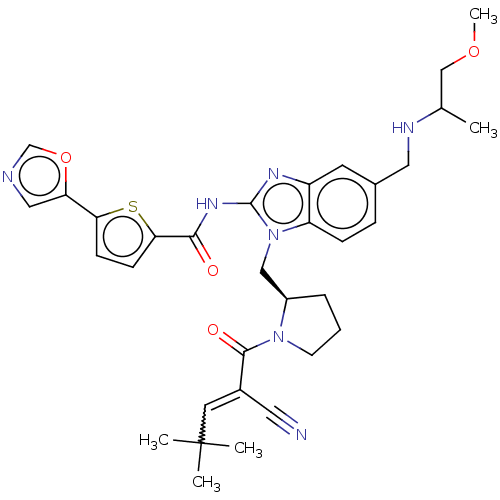 (N-1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384536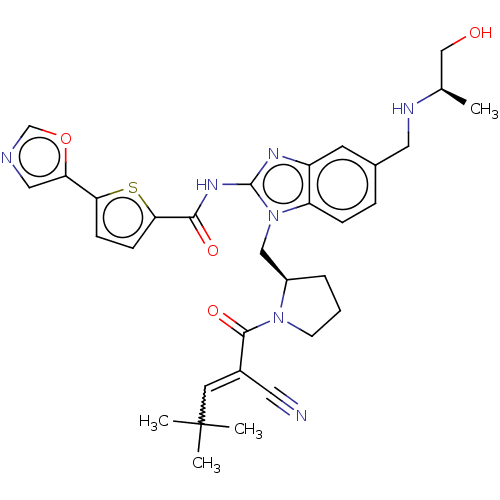 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384535 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384534 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384394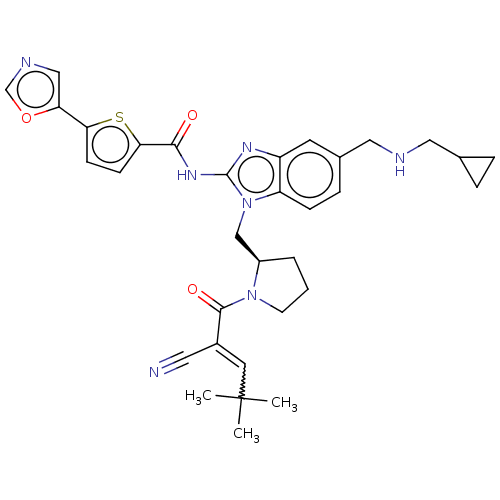 ((R)-N-(1-((1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384544 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384381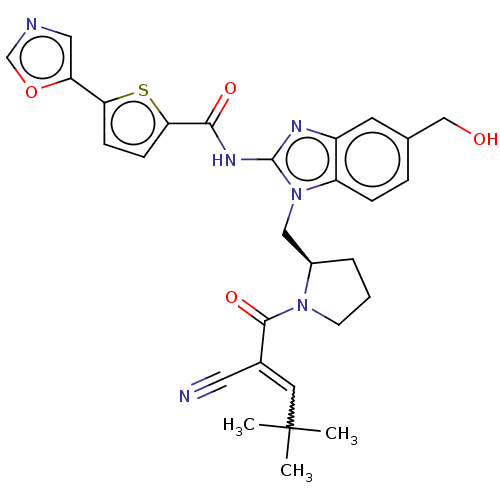 ((R)-N-(1-((1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384536 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM384381 ((R)-N-(1-((1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384533 (N-(1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384537 (N-1-(((R)-1-(2-cyano-4,4-dimethylpent-2-enoyl)pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM384394 ((R)-N-(1-((1-(2-cyano-4,4-dimethylpent-2-enoyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description The ability of the compounds of the present disclosure to inhibit ITK was measured using the Caliper assay format, which is an electrophoretic separa... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q21Z46RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||