

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047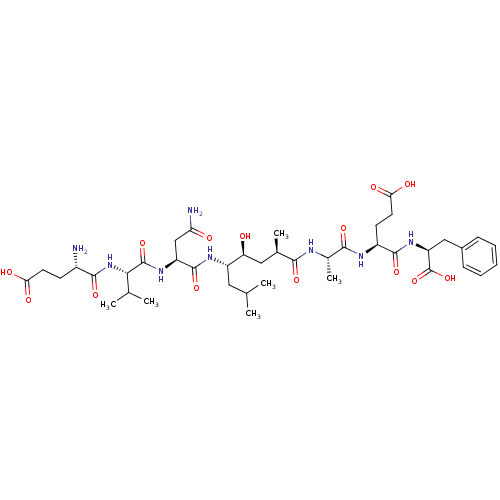 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16250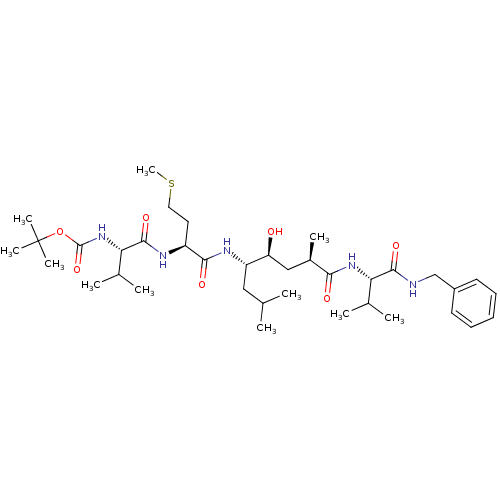 (CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -51.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16777 (Substrate-based BACE-1 inhibitor, 16 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | -48.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16781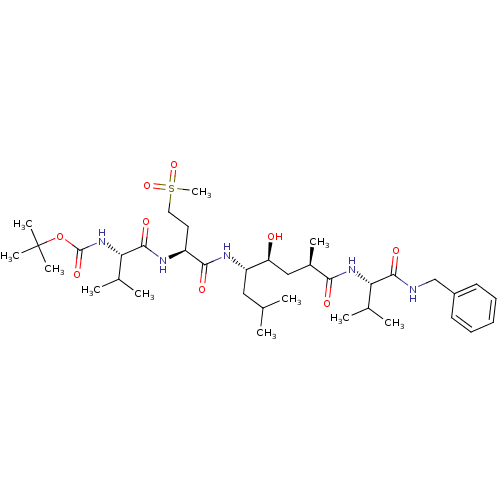 (Substrate-based BACE-1 inhibitor, 23 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16779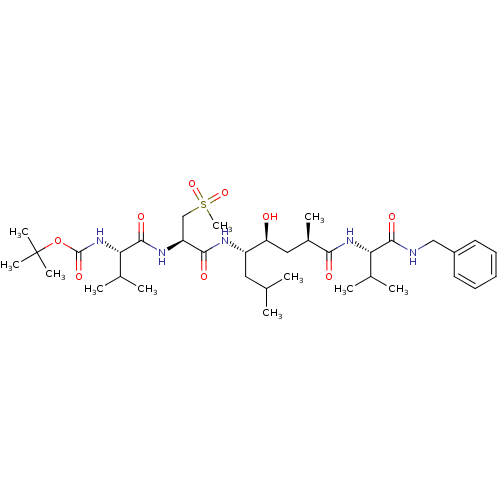 (Substrate-based BACE-1 inhibitor, 18 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.40 | -47.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16772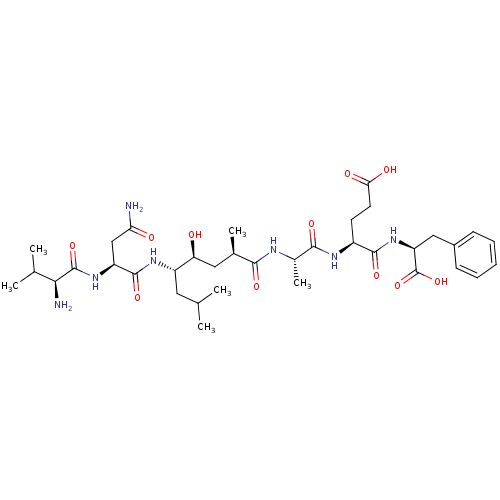 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | -44.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16778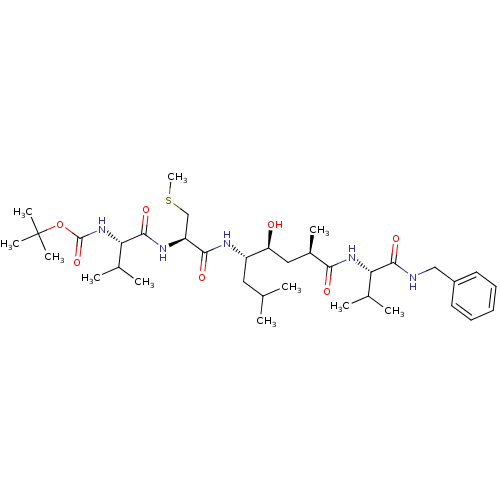 (Substrate-based BACE-1 inhibitor, 17 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 50.1 | -43.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16776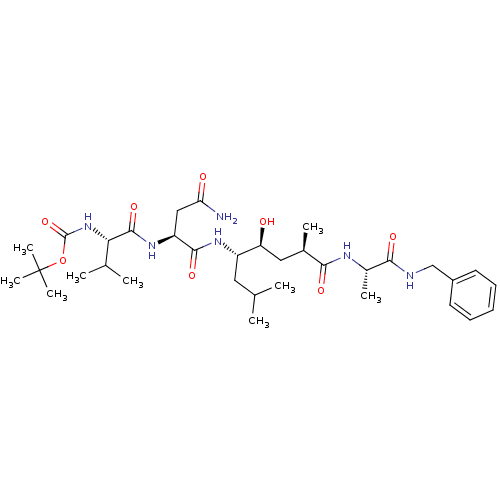 (Substrate-based BACE-1 inhibitor, 15 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61.4 | -42.8 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16775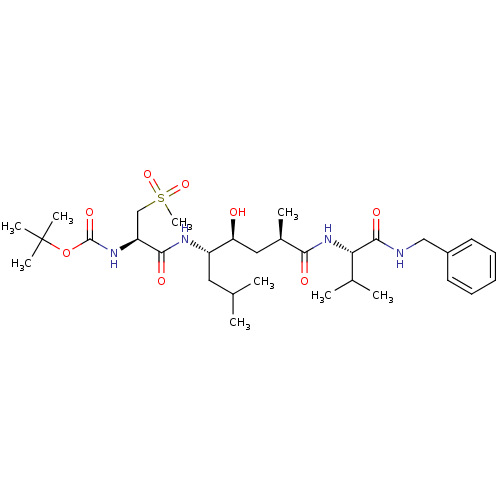 (CHEMBL273916 | Substrate-based BACE-1 inhibitor, 1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13E+3 | -35.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16774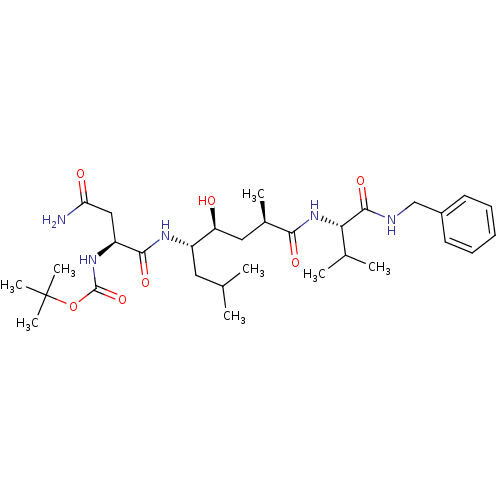 (Substrate-based BACE-1 inhibitor, 13 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.13E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16780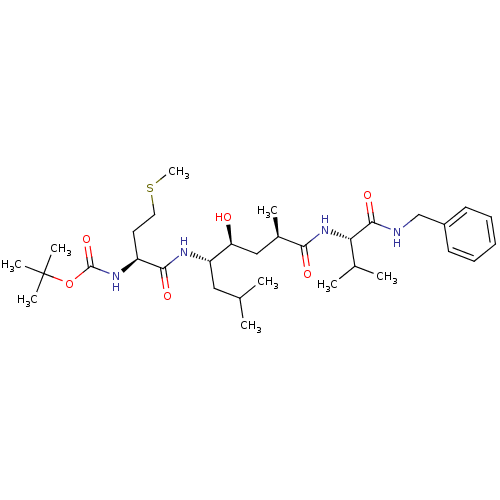 (Substrate-based BACE-1 inhibitor, 19 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.81E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16782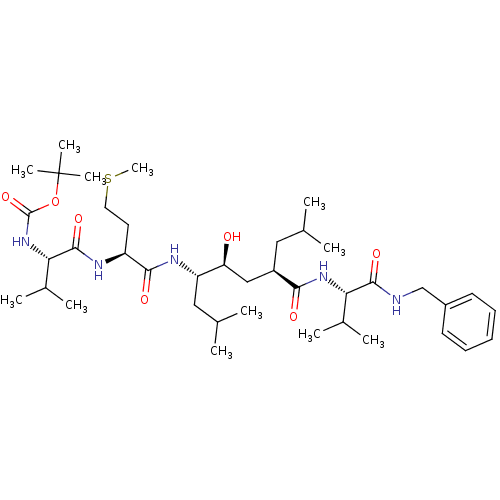 (Substrate-based BACE-1 inhibitor, 24 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.05E+4 | -29.6 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16773 (Substrate-based BACE-1 inhibitor, 12 | tert-butyl ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.24E+4 | -27.6 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
University of Illinois at Chicago | Assay Description Enzyme activities were assayed by monitoring the hydrolysis of substrate in the presence or absence of inhibitor compounds. The hydrolysis was record... | J Med Chem 44: 2865-8 (2001) Article DOI: 10.1021/jm0101803 BindingDB Entry DOI: 10.7270/Q2BG2M7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||