

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18382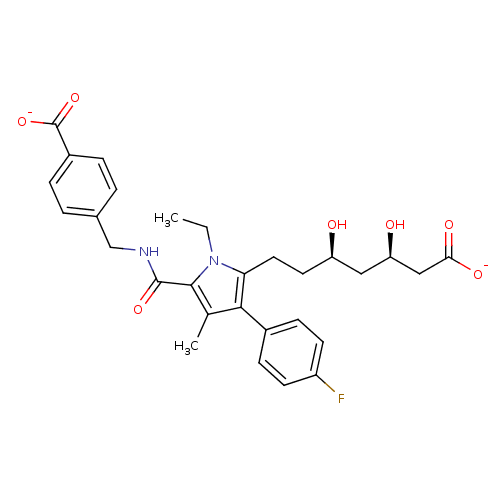 (Alkyl substituted pyrrole compound, 29f | disodium...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18387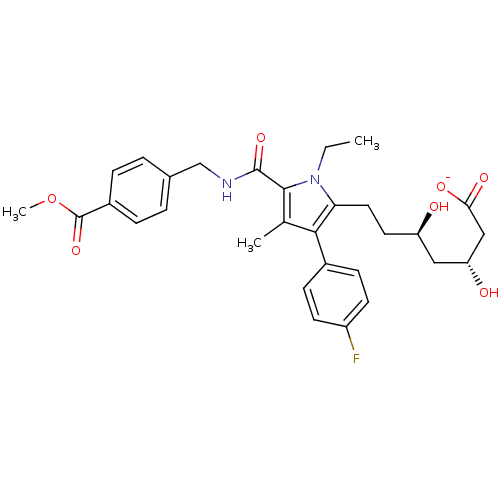 (Alkyl substituted pyrrole compound, 29d | sodium (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18384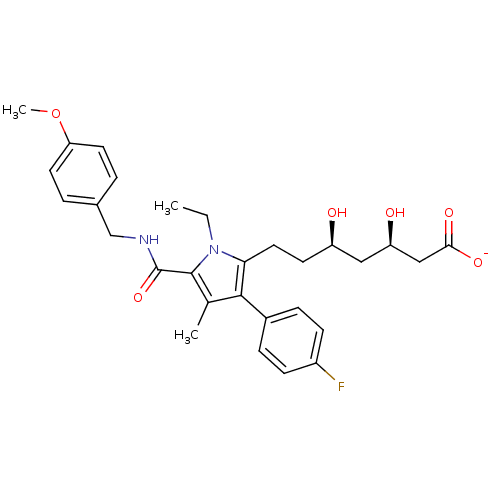 (Alkyl substituted pyrrole compound, 29c | sodium (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18381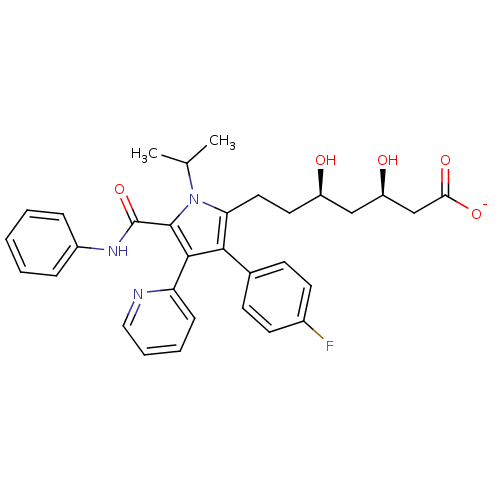 (Pyridyl substituted pyrrole compound, 14 | sodium ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18386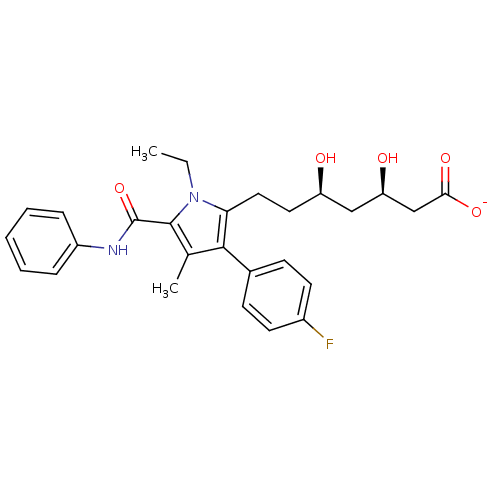 (Alkyl substituted pyrrole compound, 29b | sodium (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18376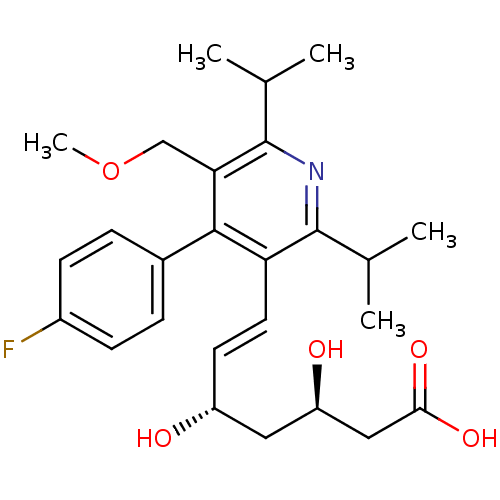 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-5-(methoxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18372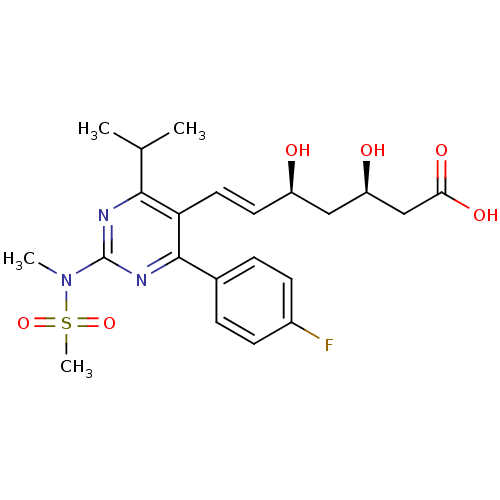 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18380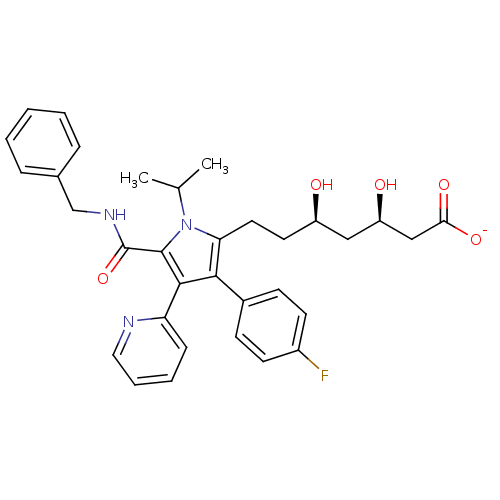 (Pyridyl substituted pyrrole compound, 15 | sodium ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18383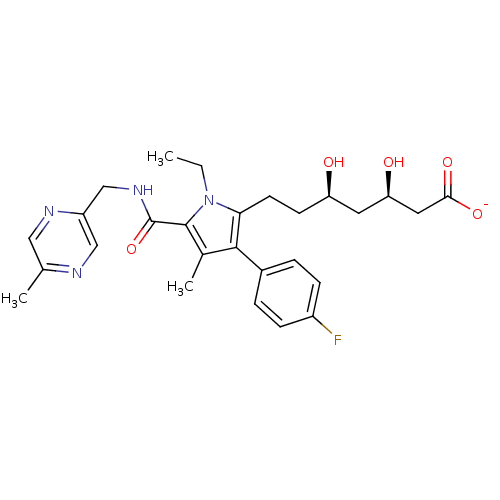 (Alkyl substituted pyrrole compound, 29e | sodium (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18379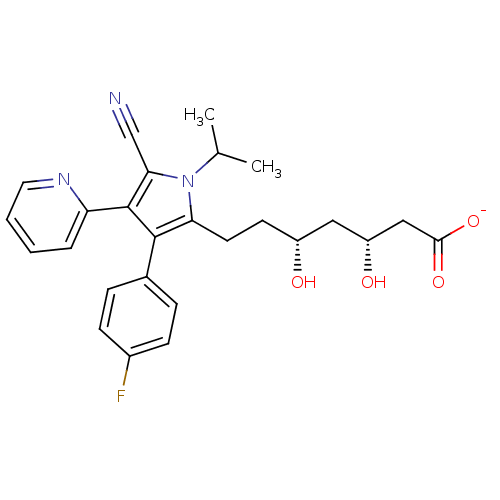 (Pyridyl substituted pyrrole compound, 11 | sodium ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18377 (Pyridyl substituted pyrrole compound, 12 | sodium ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18374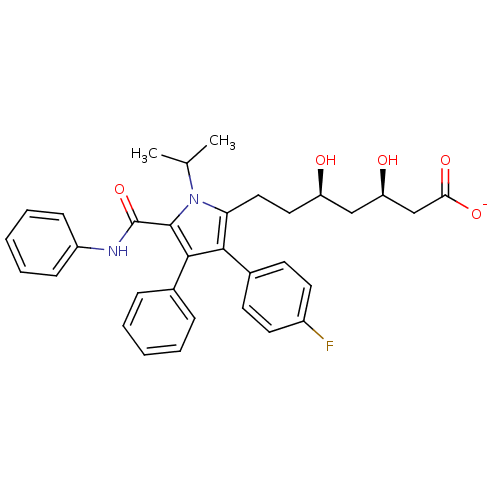 (CHEMBL394937 | Pyrrole-based compound, 30 | sodium...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18375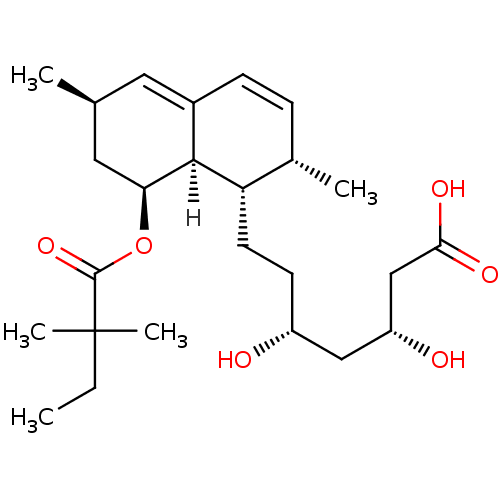 ((3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18378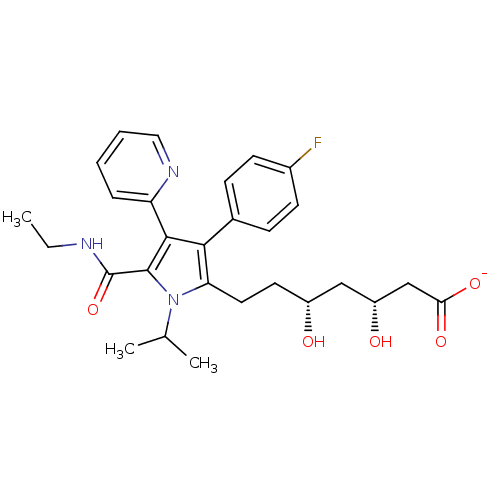 (Pyridyl substituted pyrrole compound, 13 | sodium ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM18385 (Alkyl substituted pyrrole compound, 29a | sodium (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer | Assay Description Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... | Bioorg Med Chem 15: 5576-89 (2007) Article DOI: 10.1016/j.bmc.2007.05.031 BindingDB Entry DOI: 10.7270/Q23J3B7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||