 Found 35 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50043382
Found 35 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50043382 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440280
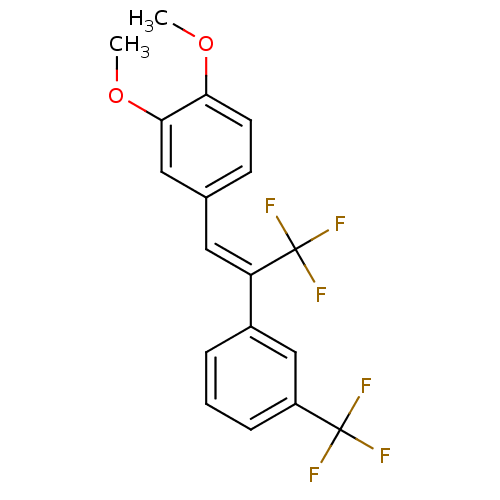
(CHEMBL489688)Show SMILES COc1ccc(\C=C(\c2cccc(c2)C(F)(F)F)C(F)(F)F)cc1OC Show InChI InChI=1S/C18H14F6O2/c1-25-15-7-6-11(9-16(15)26-2)8-14(18(22,23)24)12-4-3-5-13(10-12)17(19,20)21/h3-10H,1-2H3/b14-8- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440268

(2-(3,5-Dimethoxystyryl)Naphthalene | CHEMBL473724)Show InChI InChI=1S/C20H18O2/c1-21-19-12-16(13-20(14-19)22-2)8-7-15-9-10-17-5-3-4-6-18(17)11-15/h3-14H,1-2H3/b8-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247277
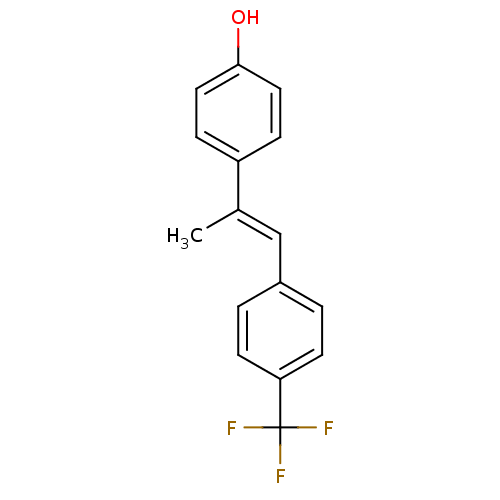
(4-(1-(4-(trifluoromethyl)phenyl)prop-1-en-2-yl)phe...)Show InChI InChI=1S/C16H13F3O/c1-11(13-4-8-15(20)9-5-13)10-12-2-6-14(7-3-12)16(17,18)19/h2-10,20H,1H3/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23933
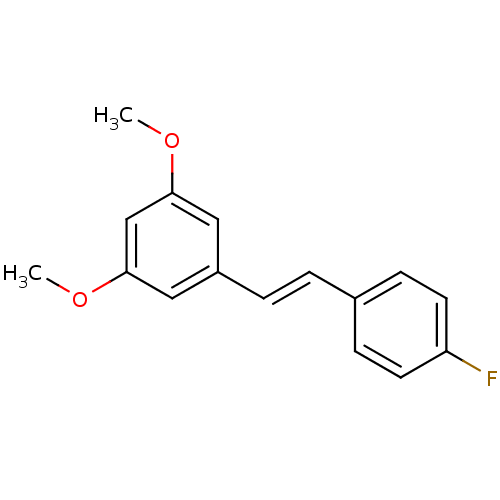
(1-[(E)-2-(4-fluorophenyl)ethenyl]-3,5-dimethoxyben...)Show InChI InChI=1S/C16H15FO2/c1-18-15-9-13(10-16(11-15)19-2)4-3-12-5-7-14(17)8-6-12/h3-11H,1-2H3/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247278
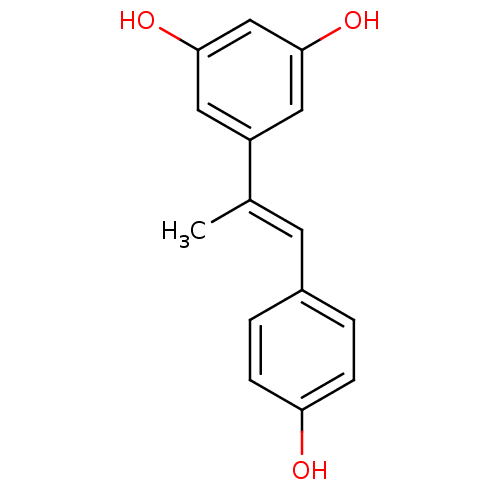
(5-(1-(4-hydroxyphenyl)prop-1-en-2-yl)benzene-1,3-d...)Show InChI InChI=1S/C15H14O3/c1-10(6-11-2-4-13(16)5-3-11)12-7-14(17)9-15(18)8-12/h2-9,16-18H,1H3/b10-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440277
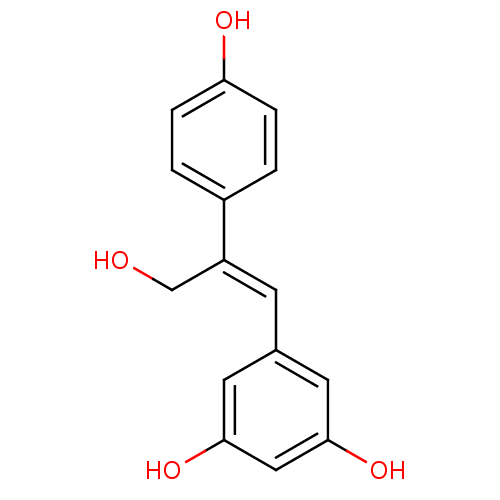
(CHEMBL2426745)Show InChI InChI=1S/C15H14O4/c16-9-12(11-1-3-13(17)4-2-11)5-10-6-14(18)8-15(19)7-10/h1-8,16-19H,9H2/b12-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50131688
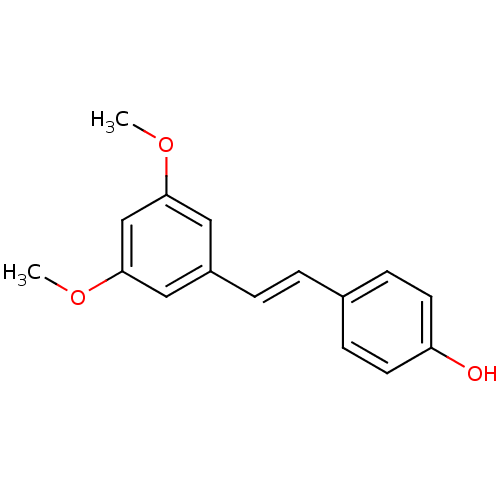
((E)-4-(3,5-dimethoxystyryl)phenol | 3,5-Dimethoxy-...)Show InChI InChI=1S/C16H16O3/c1-18-15-9-13(10-16(11-15)19-2)4-3-12-5-7-14(17)8-6-12/h3-11,17H,1-2H3/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440265
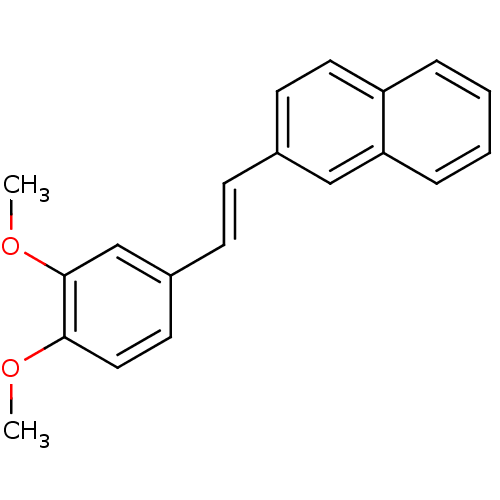
(CHEMBL2426739)Show InChI InChI=1S/C20H18O2/c1-21-19-12-10-16(14-20(19)22-2)8-7-15-9-11-17-5-3-4-6-18(17)13-15/h3-14H,1-2H3/b8-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM76669
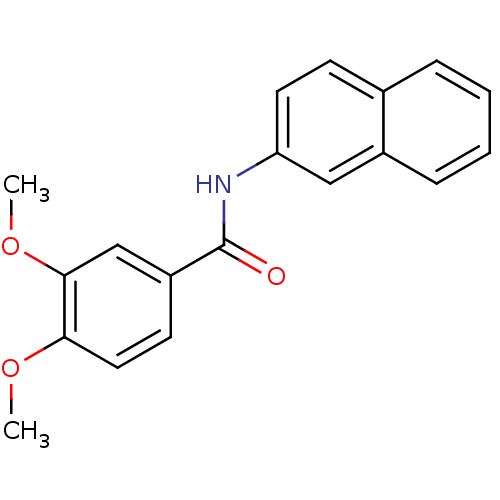
(3,4-dimethoxy-N-(2-naphthalenyl)benzamide | 3,4-di...)Show InChI InChI=1S/C19H17NO3/c1-22-17-10-8-15(12-18(17)23-2)19(21)20-16-9-7-13-5-3-4-6-14(13)11-16/h3-12H,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440278

(CHEMBL2426744)Show InChI InChI=1S/C15H11NO3/c16-9-12(11-1-3-13(17)4-2-11)5-10-6-14(18)8-15(19)7-10/h1-8,17-19H/b12-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50045921

(3,4,4'-trihydroxy-trans-stilbene | 4-(4-hydroxysty...)Show InChI InChI=1S/C14H12O3/c15-12-6-3-10(4-7-12)1-2-11-5-8-13(16)14(17)9-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440282
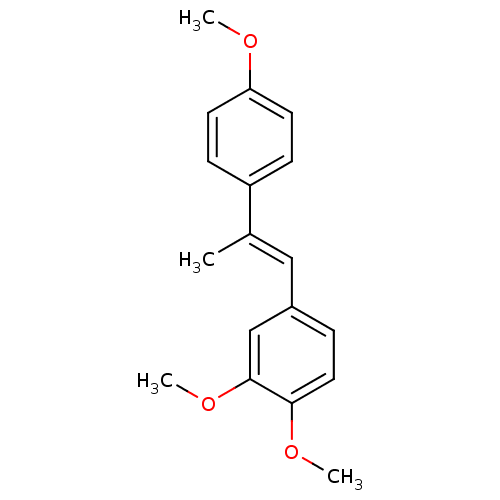
(CHEMBL2426740)Show InChI InChI=1S/C18H20O3/c1-13(15-6-8-16(19-2)9-7-15)11-14-5-10-17(20-3)18(12-14)21-4/h5-12H,1-4H3/b13-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440267
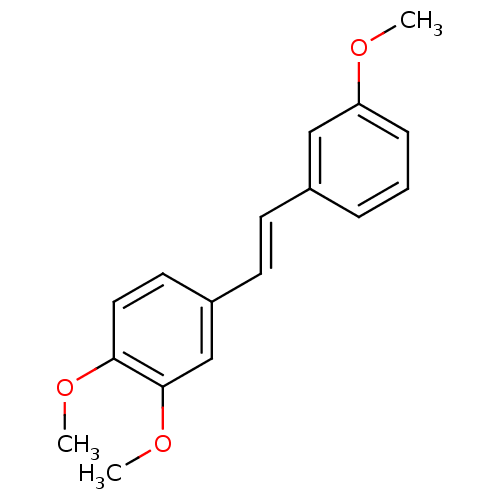
(CHEMBL473725)Show InChI InChI=1S/C17H18O3/c1-18-15-6-4-5-13(11-15)7-8-14-9-10-16(19-2)17(12-14)20-3/h4-12H,1-3H3/b8-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440270
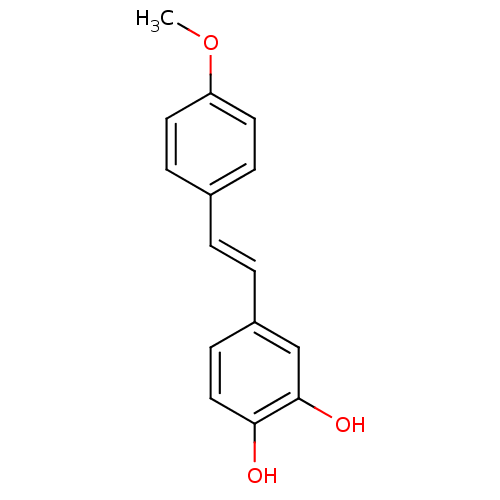
(CHEMBL215432)Show InChI InChI=1S/C15H14O3/c1-18-13-7-4-11(5-8-13)2-3-12-6-9-14(16)15(17)10-12/h2-10,16-17H,1H3/b3-2+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440275
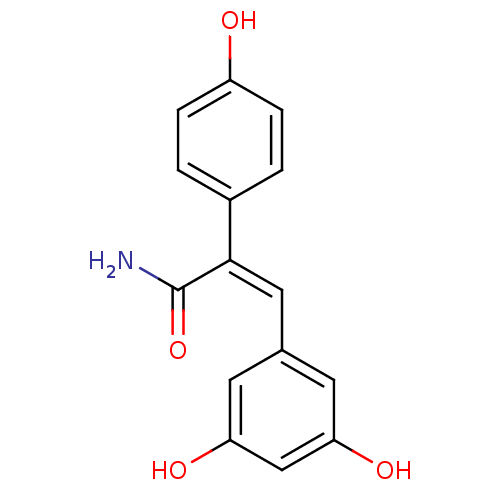
(CHEMBL2426747)Show InChI InChI=1S/C15H13NO4/c16-15(20)14(10-1-3-11(17)4-2-10)7-9-5-12(18)8-13(19)6-9/h1-8,17-19H,(H2,16,20)/b14-7- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440281
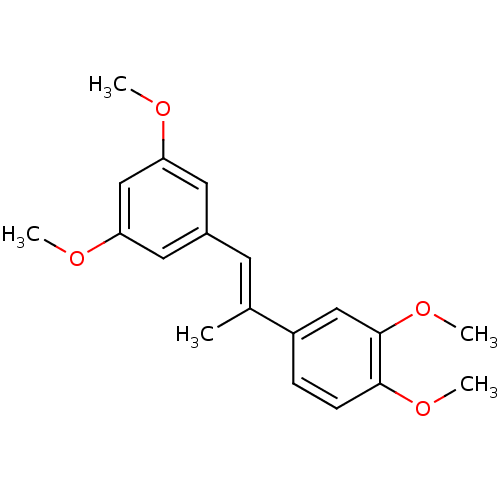
(CHEMBL2426741)Show InChI InChI=1S/C19H22O4/c1-13(15-6-7-18(22-4)19(11-15)23-5)8-14-9-16(20-2)12-17(10-14)21-3/h6-12H,1-5H3/b13-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50256049
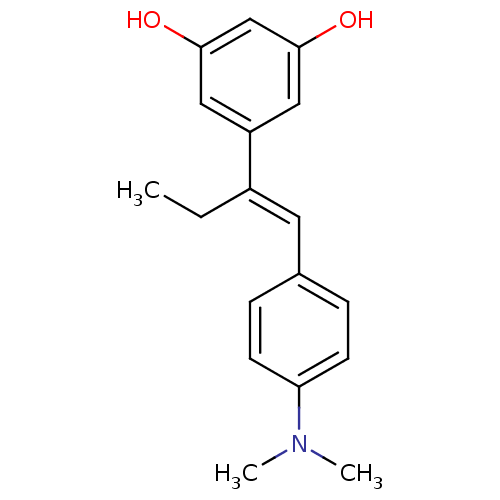
(5-(1-(4-(dimethylamino)phenyl)but-1-en-2-yl)benzen...)Show InChI InChI=1S/C18H21NO2/c1-4-14(15-10-17(20)12-18(21)11-15)9-13-5-7-16(8-6-13)19(2)3/h5-12,20-21H,4H2,1-3H3/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247186

(5-(2-(4-methoxyphenyl)prop-1-enyl)benzene-1,3-diol...)Show InChI InChI=1S/C16H16O3/c1-11(13-3-5-16(19-2)6-4-13)7-12-8-14(17)10-15(18)9-12/h3-10,17-18H,1-2H3/b11-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440266

(CHEMBL474126)Show InChI InChI=1S/C18H21NO2/c1-19(2)16-10-7-14(8-11-16)5-6-15-9-12-17(20-3)18(13-15)21-4/h5-13H,1-4H3/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247233

(1,3-dimethoxy-5-(3,3,3-trifluoro-1-(4-methoxypheny...)Show SMILES COc1ccc(\C=C(\c2cc(OC)cc(OC)c2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H17F3O3/c1-22-14-6-4-12(5-7-14)8-17(18(19,20)21)13-9-15(23-2)11-16(10-13)24-3/h4-11H,1-3H3/b17-8- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440269

(CHEMBL474264)Show InChI InChI=1S/C15H11F3O2/c16-15(17,18)12-6-3-10(4-7-12)1-2-11-5-8-13(19)14(20)9-11/h1-9,19-20H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23928
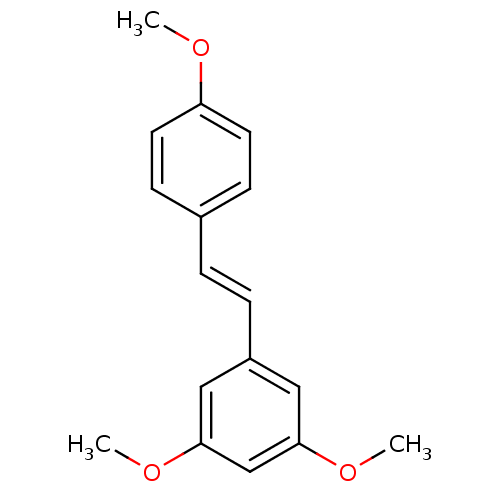
(1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]be...)Show InChI InChI=1S/C17H18O3/c1-18-15-8-6-13(7-9-15)4-5-14-10-16(19-2)12-17(11-14)20-3/h4-12H,1-3H3/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440279

(CHEMBL470939)Show InChI InChI=1S/C20H24O4/c1-6-15(16-7-8-19(23-4)20(12-16)24-5)9-14-10-17(21-2)13-18(11-14)22-3/h7-13H,6H2,1-5H3/b15-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440271
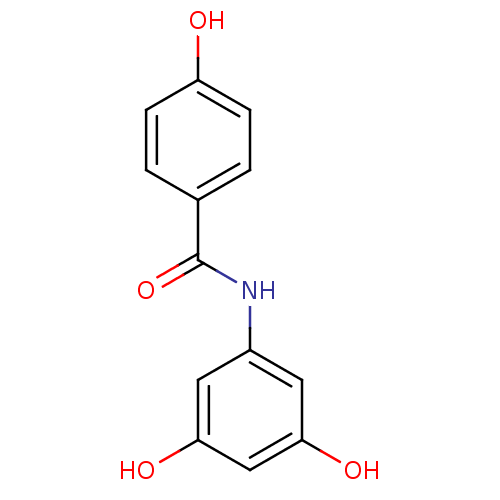
(CHEMBL2426751)Show InChI InChI=1S/C13H11NO4/c15-10-3-1-8(2-4-10)13(18)14-9-5-11(16)7-12(17)6-9/h1-7,15-17H,(H,14,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440283
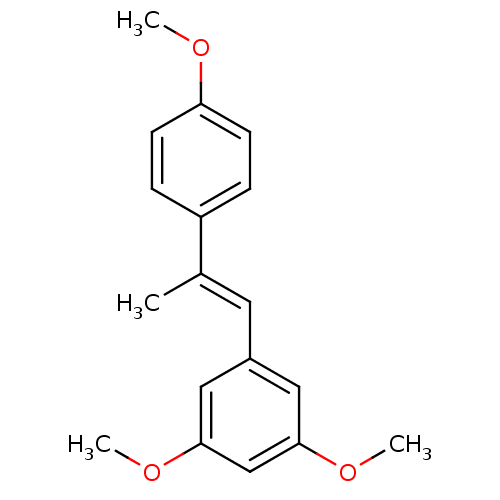
(CHEMBL474936)Show InChI InChI=1S/C18H20O3/c1-13(15-5-7-16(19-2)8-6-15)9-14-10-17(20-3)12-18(11-14)21-4/h5-12H,1-4H3/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247182
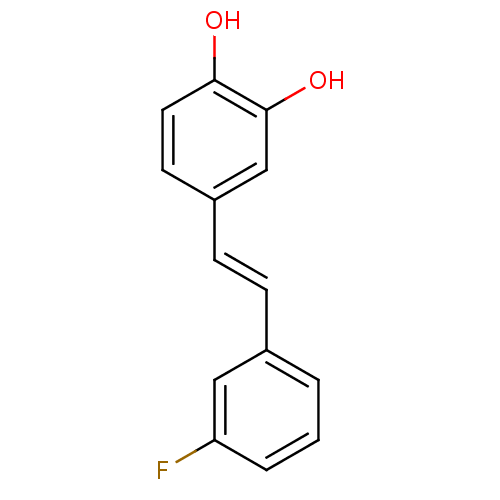
(4-(3-fluorostyryl)benzene-1,2-diol | CHEMBL464755)Show InChI InChI=1S/C14H11FO2/c15-12-3-1-2-10(8-12)4-5-11-6-7-13(16)14(17)9-11/h1-9,16-17H/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440276
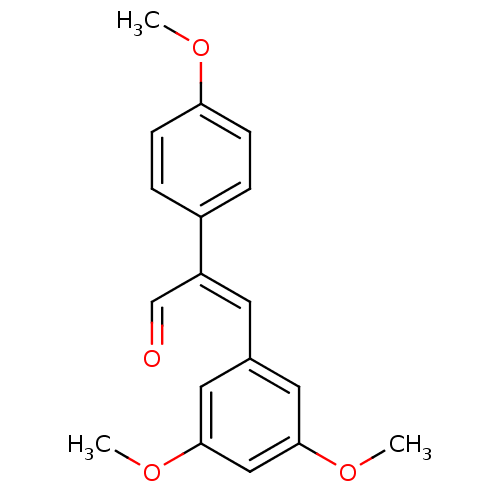
(CHEMBL2426746)Show InChI InChI=1S/C18H18O4/c1-20-16-6-4-14(5-7-16)15(12-19)8-13-9-17(21-2)11-18(10-13)22-3/h4-12H,1-3H3/b15-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440274

(CHEMBL2426748)Show InChI InChI=1S/C15H12O4/c16-9-12(11-1-3-13(17)4-2-11)5-10-6-14(18)8-15(19)7-10/h1-9,17-19H/b12-5- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440273
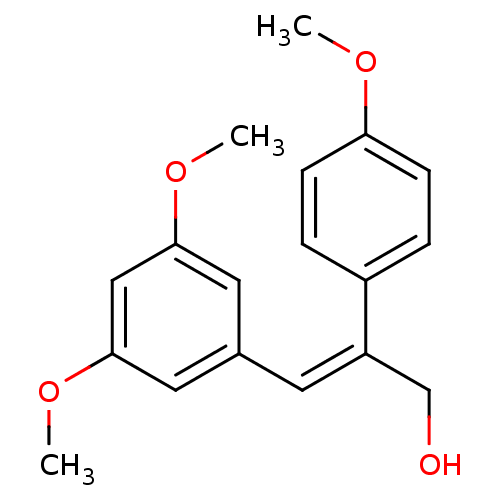
(CHEMBL2426749)Show InChI InChI=1S/C18H20O4/c1-20-16-6-4-14(5-7-16)15(12-19)8-13-9-17(21-2)11-18(10-13)22-3/h4-11,19H,12H2,1-3H3/b15-8- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50440272

(CHEMBL2426750)Show InChI InChI=1S/C13H11NO4/c15-10-3-1-9(2-4-10)14-13(18)8-5-11(16)7-12(17)6-8/h1-7,15-17H,(H,14,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50145707

((Z)-3-(3,5-Dimethoxy-phenyl)-2-(4-methoxy-phenyl)-...)Show InChI InChI=1S/C18H17NO3/c1-20-16-6-4-14(5-7-16)15(12-19)8-13-9-17(21-2)11-18(10-13)22-3/h4-11H,1-3H3/b15-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247189
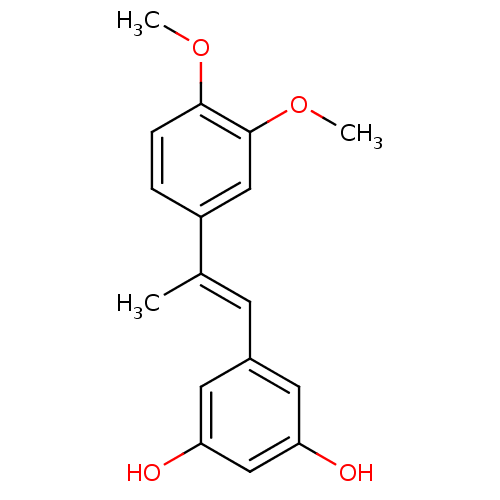
(5-(2-(3,4-dimethoxyphenyl)prop-1-enyl)benzene-1,3-...)Show InChI InChI=1S/C17H18O4/c1-11(6-12-7-14(18)10-15(19)8-12)13-4-5-16(20-2)17(9-13)21-3/h4-10,18-19H,1-3H3/b11-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50085526
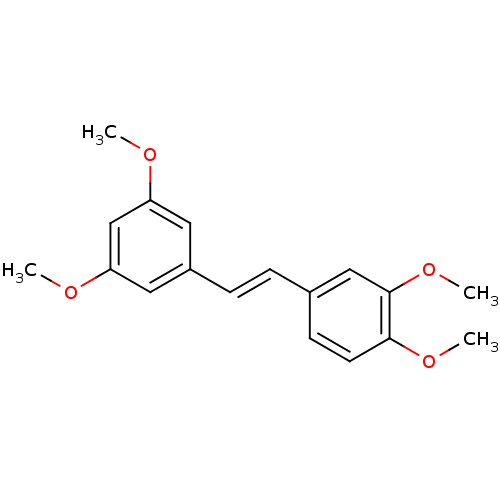
((E)-3,5,30,40-Tetramethoxystilbene | 4-[(E)-2-(3,5...)Show InChI InChI=1S/C18H20O4/c1-19-15-9-14(10-16(12-15)20-2)6-5-13-7-8-17(21-3)18(11-13)22-4/h5-12H,1-4H3/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50247226
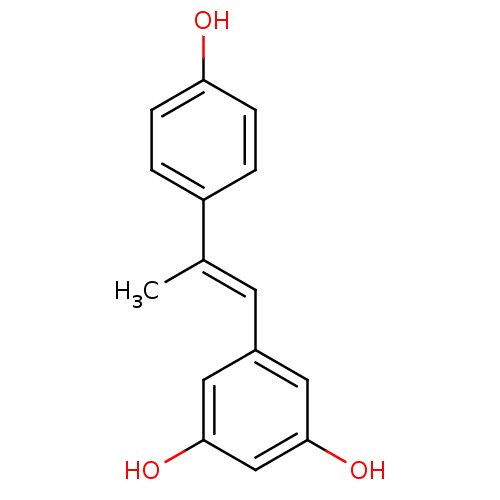
(5-(2-(4-hydroxyphenyl)prop-1-enyl)benzene-1,3-diol...)Show InChI InChI=1S/C15H14O3/c1-10(12-2-4-13(16)5-3-12)6-11-7-14(17)9-15(18)8-11/h2-9,16-18H,1H3/b10-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) using N-methyldihydronicotinamide as co-substrate |
Bioorg Med Chem 21: 6022-37 (2013)
Article DOI: 10.1016/j.bmc.2013.07.037
BindingDB Entry DOI: 10.7270/Q21R6RX0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data