

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50160077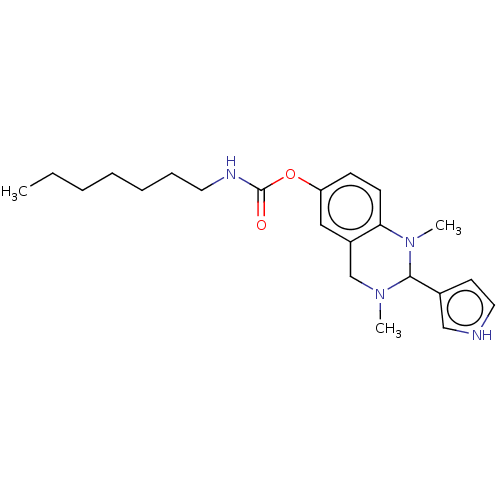 (CHEMBL3785861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 852 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50160076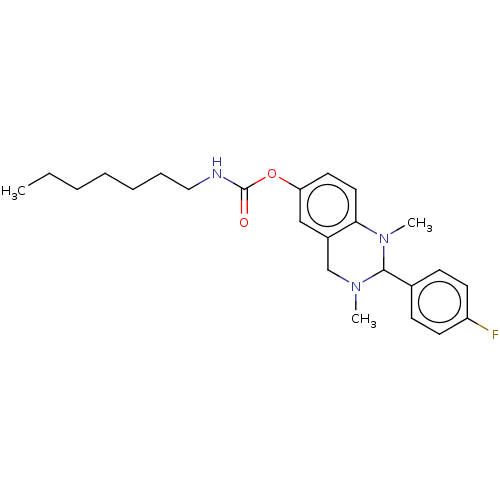 (CHEMBL3787116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t W£rzburg Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase using acetylcholine iodide as substrate preincubated for 30 mins followed by substrate addition measured aft... | J Med Chem 59: 2067-82 (2016) Article DOI: 10.1021/acs.jmedchem.5b01674 BindingDB Entry DOI: 10.7270/Q25H7J4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||