

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144637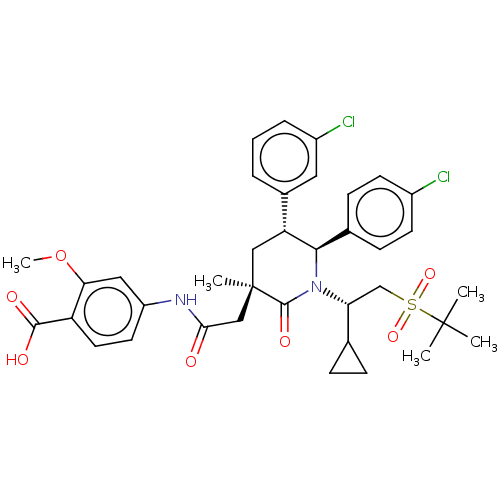 (US8952036, Ex. 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0503 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50448963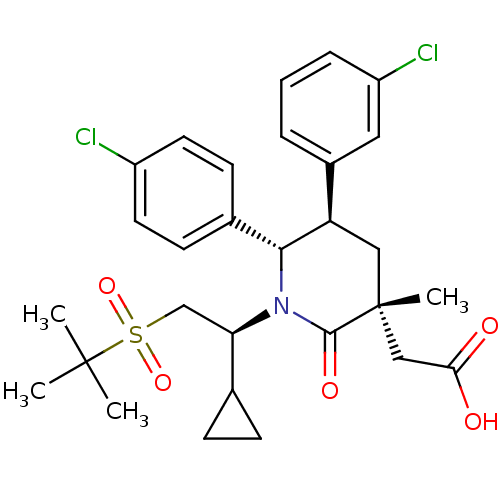 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0962 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144636 (US8952036, Ex. 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144638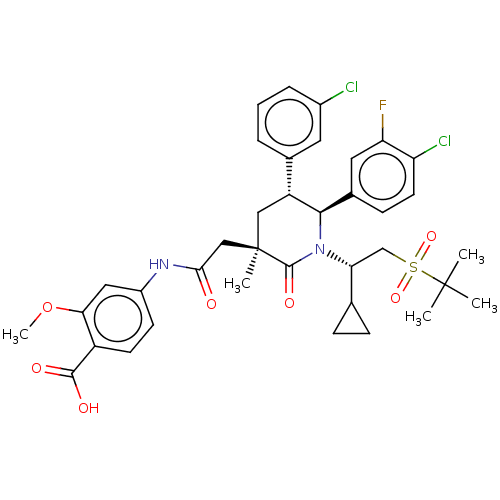 (US8952036, Ex. 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50008760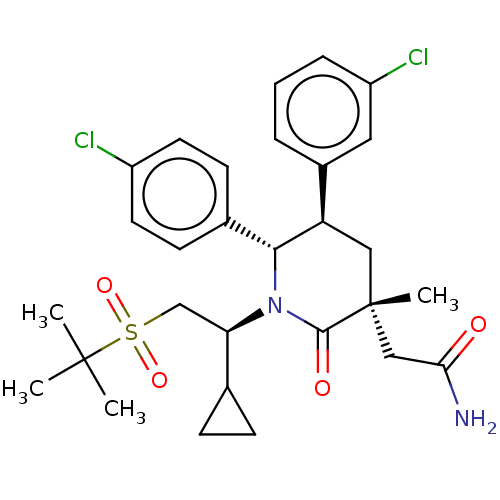 (CHEMBL3236364) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.83 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||