

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217130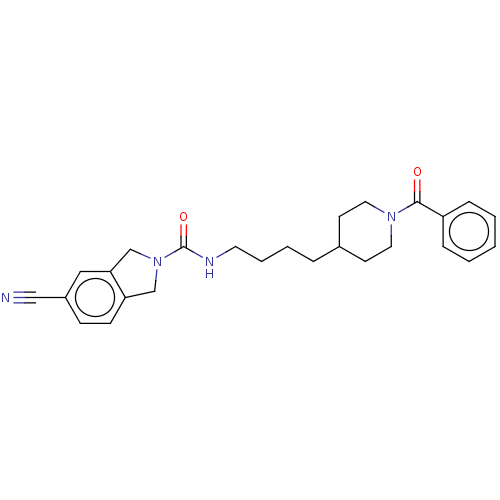 (US9302989, 451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.946 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112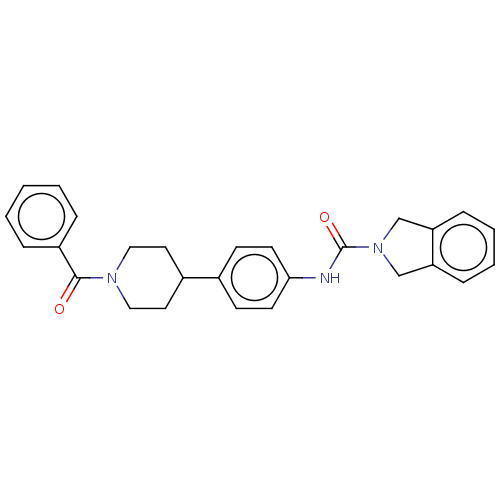 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217153 (US9302989, 563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216340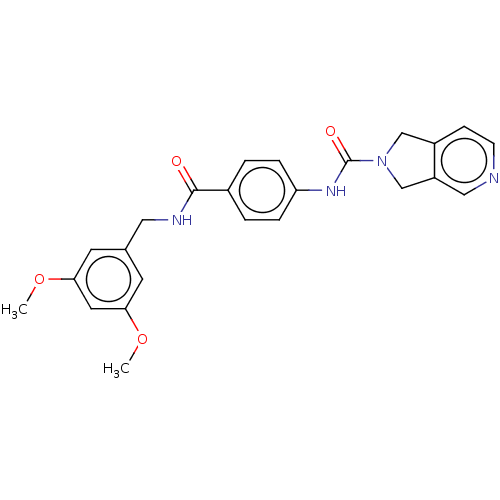 (US9302989, 285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.92 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216548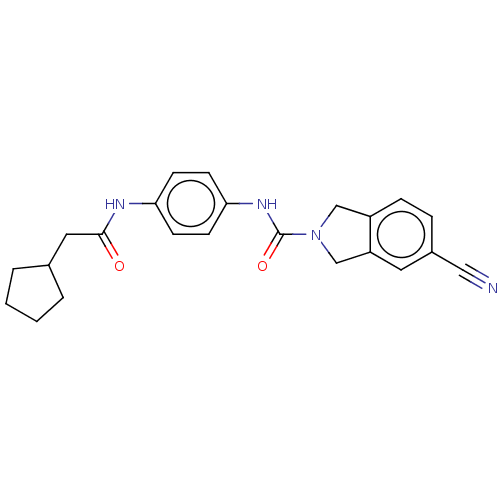 (US9302989, 564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217165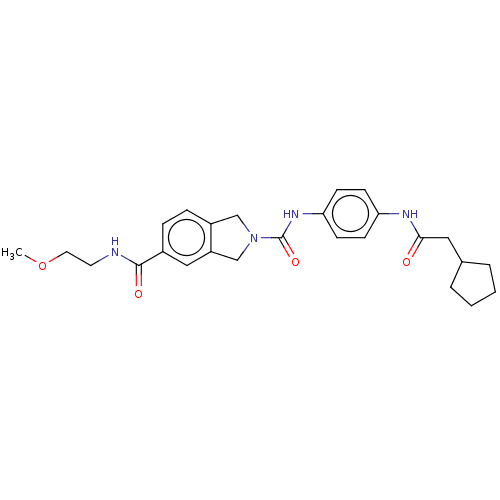 (US9302989, 582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.05 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216341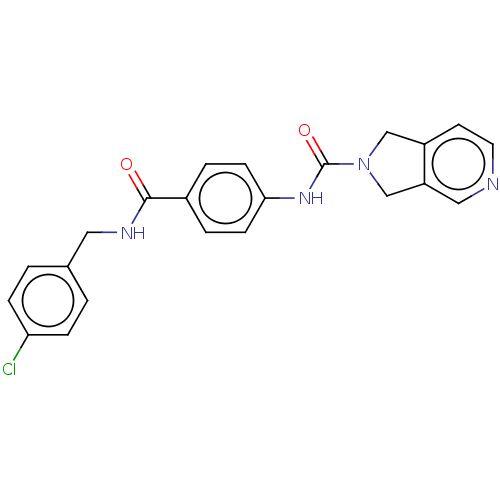 (US9302989, 286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.06 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217145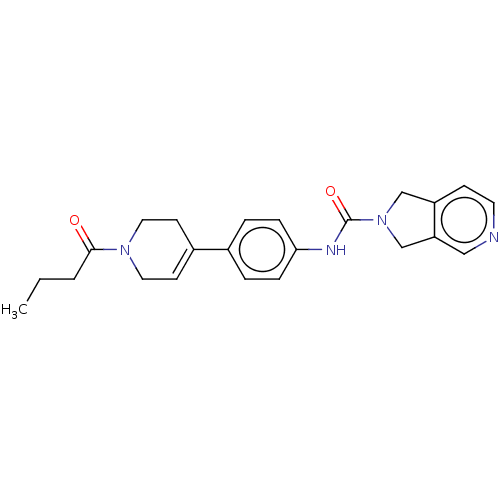 (US9302989, 543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217057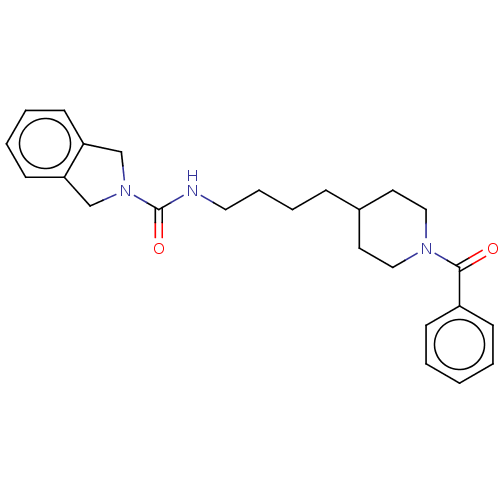 (US9302989, 349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217163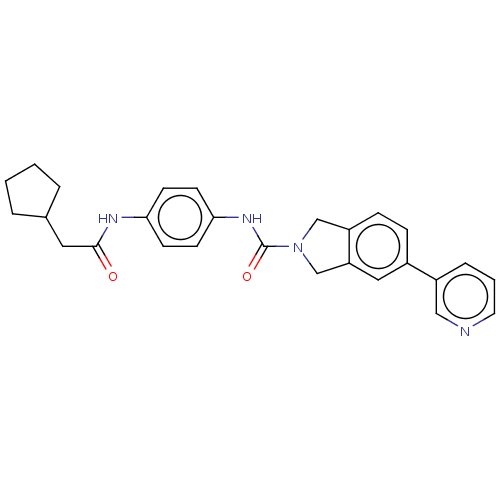 (US9302989, 580) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217144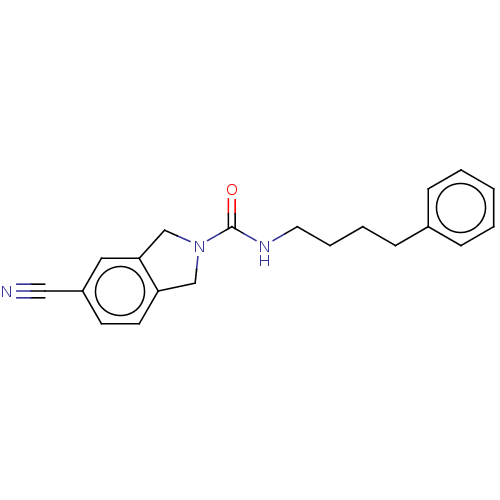 (US9302989, 542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217147 (US9302989, 545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.78 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215807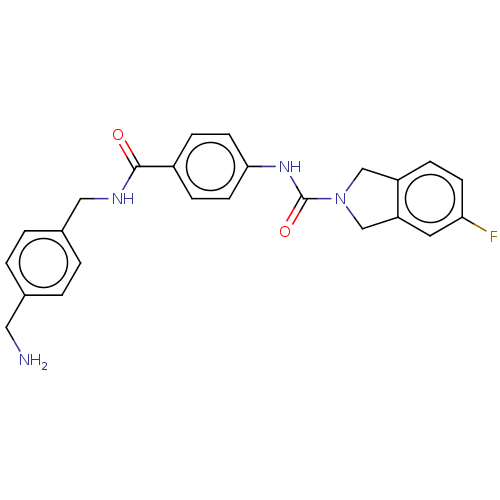 (US9302989, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.78 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217166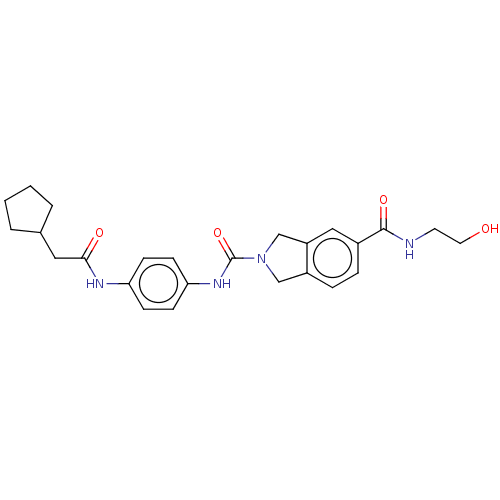 (US9302989, 583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217146 (US9302989, 544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215808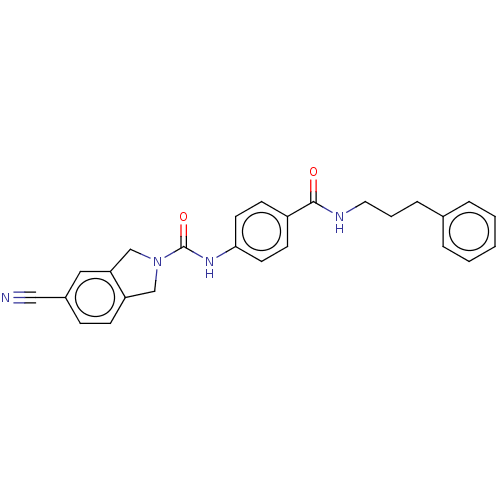 (US9302989, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217162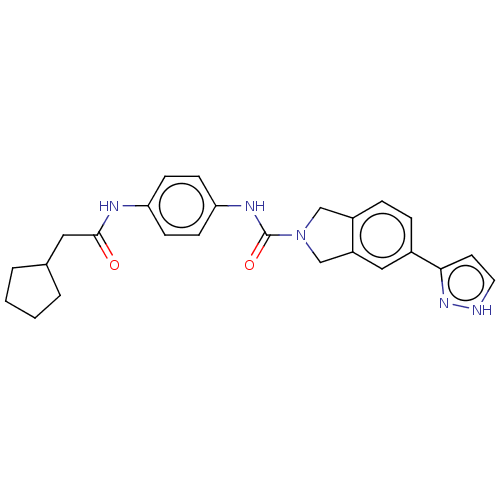 (US9302989, 579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217041 (US9302989, 333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216593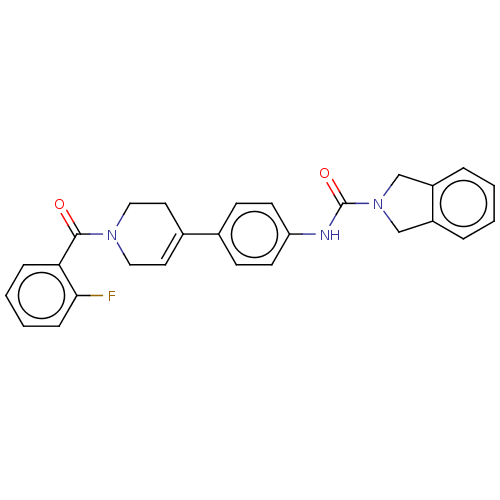 (US9302989, 473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217134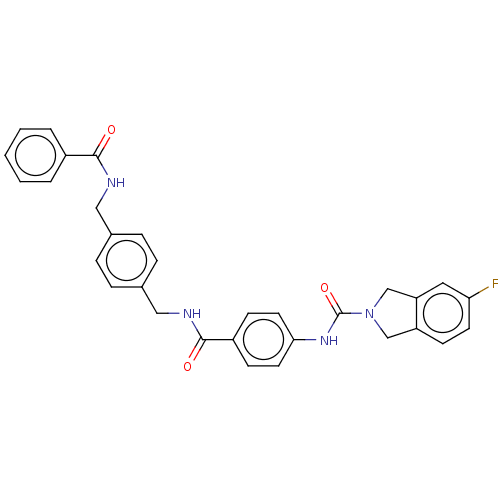 (US9302989, 497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216658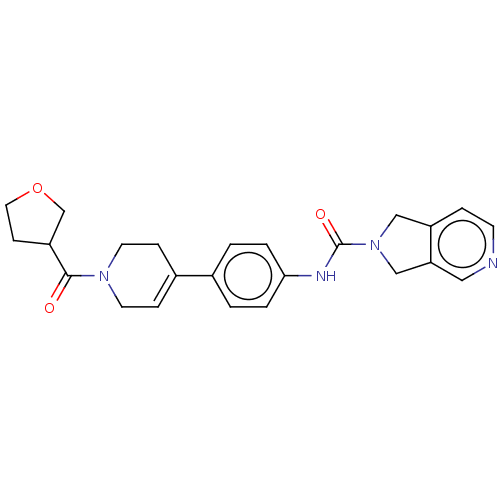 (US9302989, 546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.32 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216588 (US9302989, 468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216580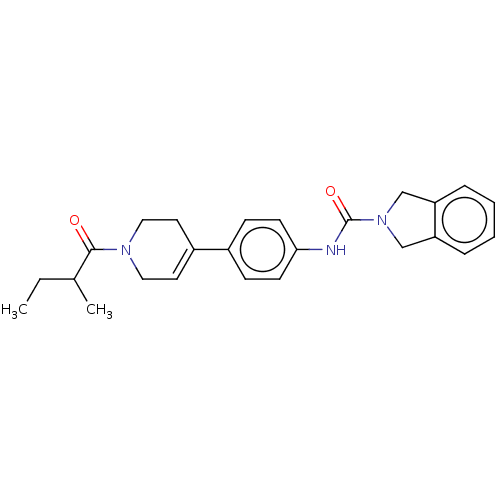 (US9302989, 459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215809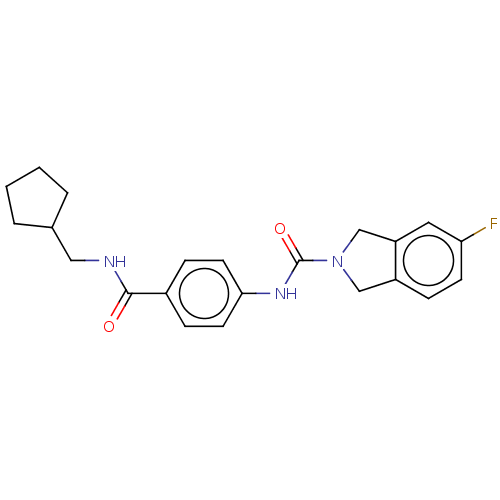 (US9302989, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217076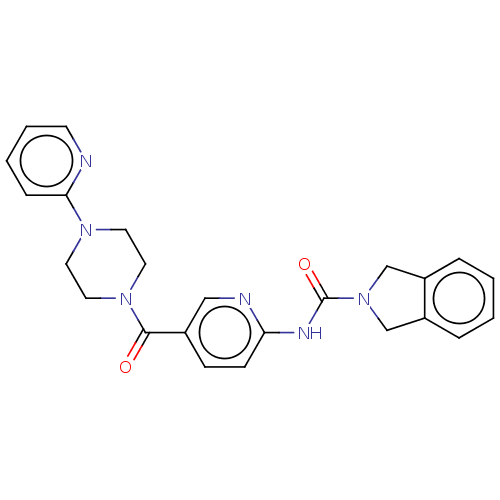 (US9302989, 368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216594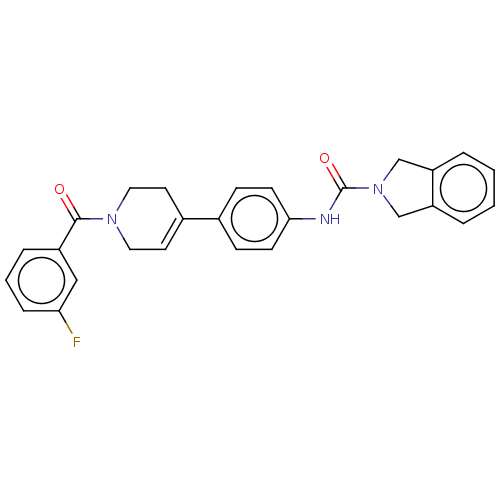 (US9302989, 474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217164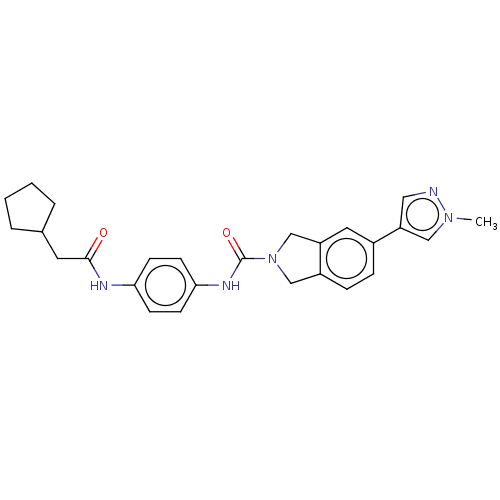 (US9302989, 581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216356 (US9302989, 554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.01 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216093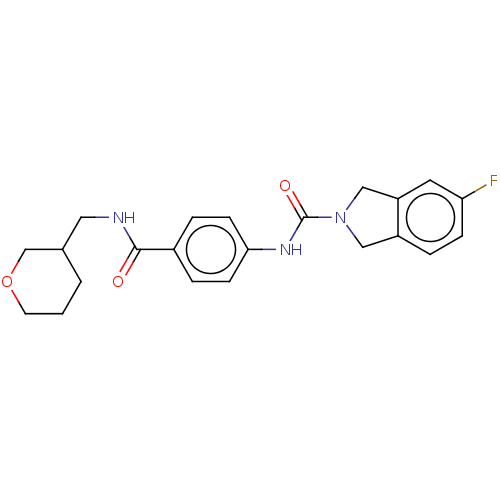 (US9302989, 568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217040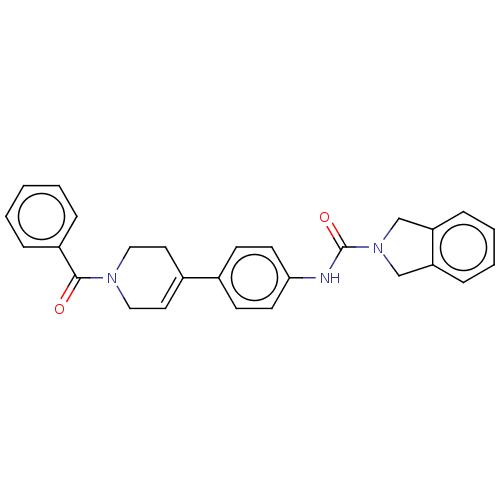 (US9302989, 332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215811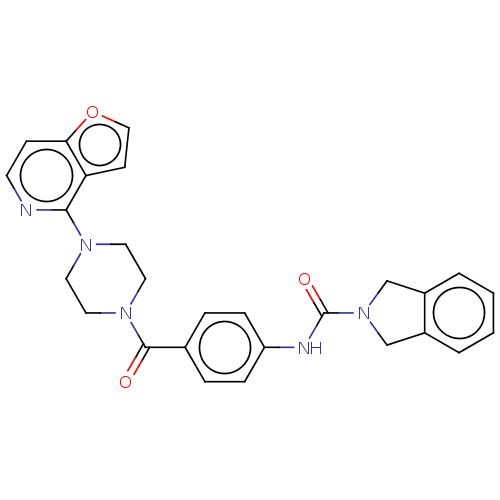 (US9302989, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216342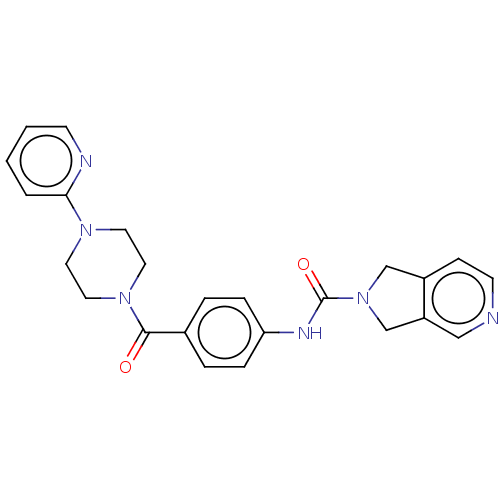 (US9302989, 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217160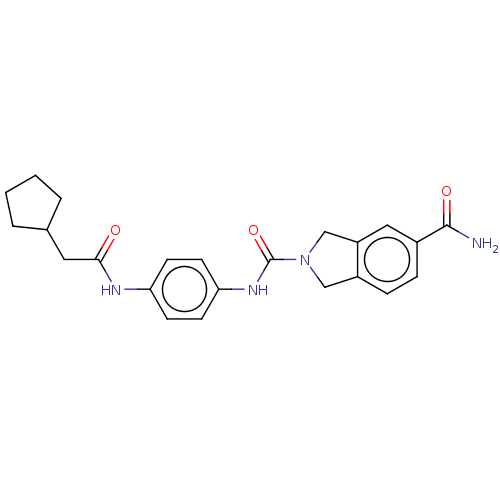 (US9302989, 577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216590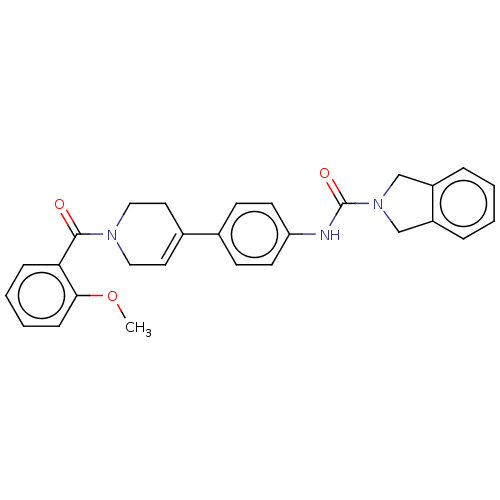 (US9302989, 470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216343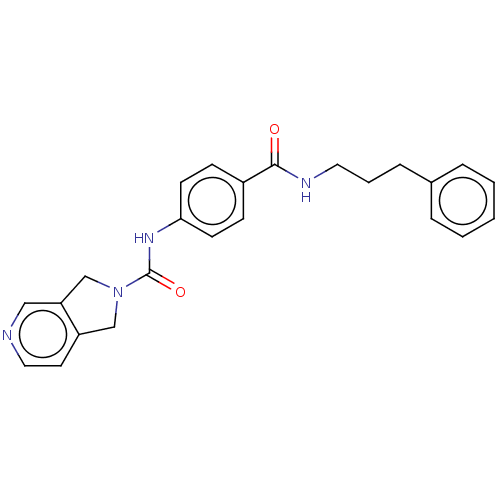 (US9302989, 288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216609 (US9302989, 491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215812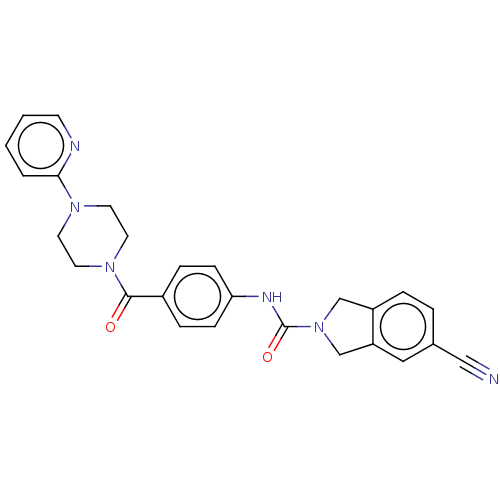 (US9302989, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216604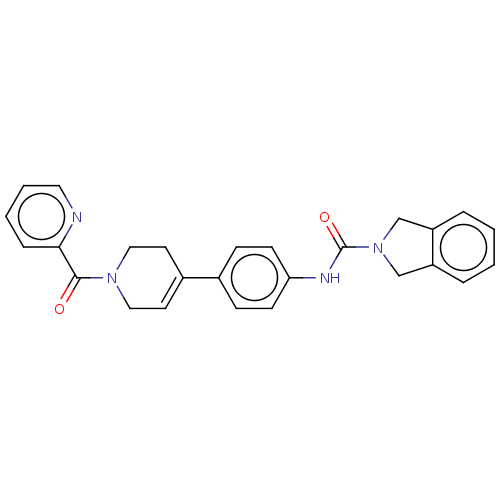 (US9302989, 486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216602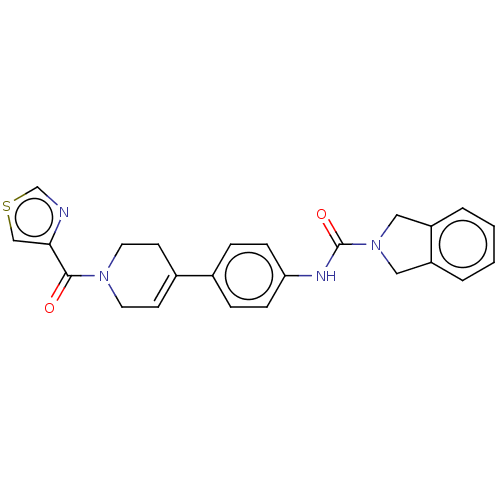 (US9302989, 483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215813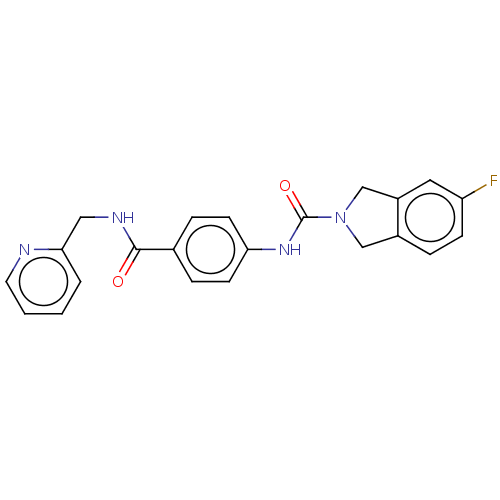 (US9302989, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.88 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215814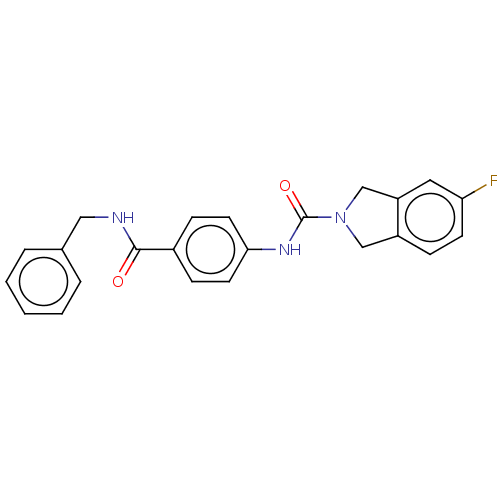 (US9302989, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.02 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216601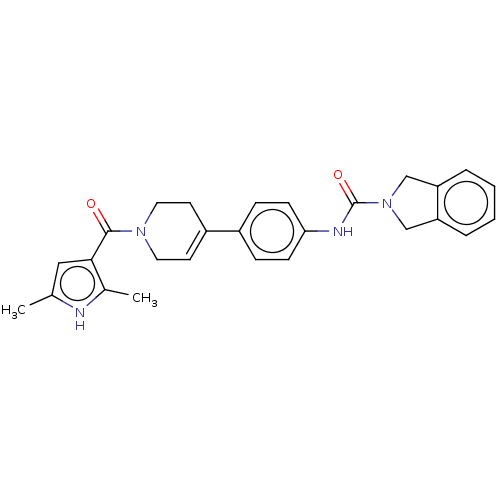 (US9302989, 482) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216546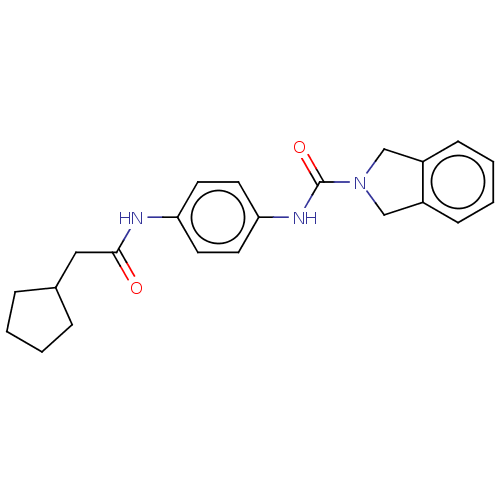 (US9302989, 311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM215815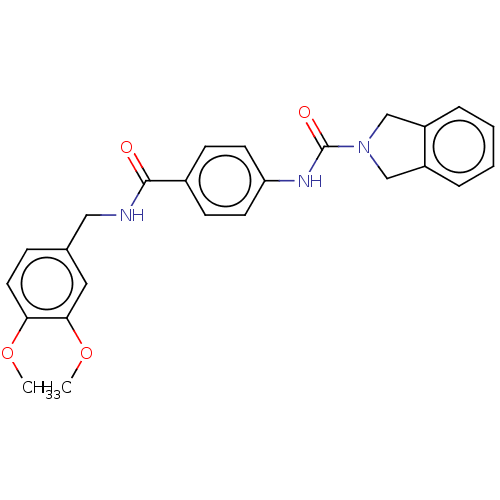 (US9302989, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217021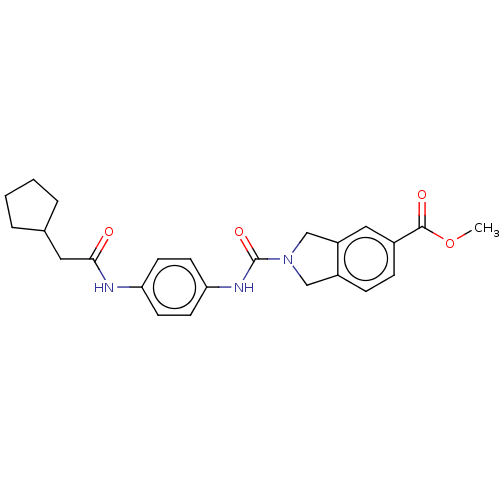 (US9302989, 313) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216603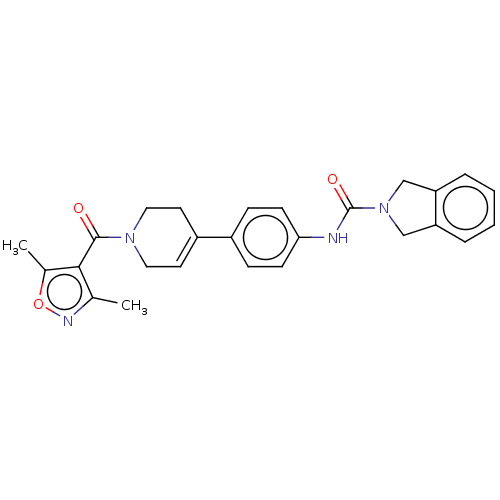 (US9302989, 485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216600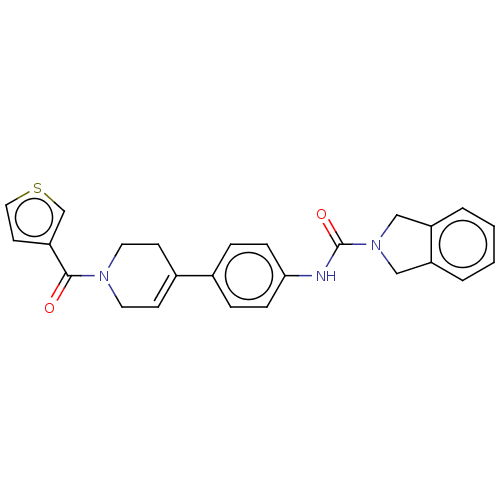 (US9302989, 480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.71 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216086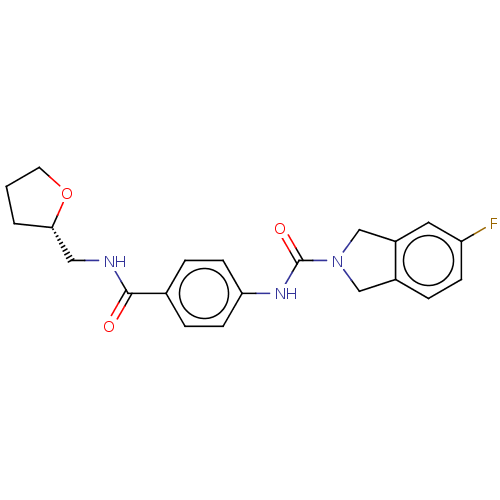 (US9302989, 435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM216589 (US9302989, 469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.97 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217039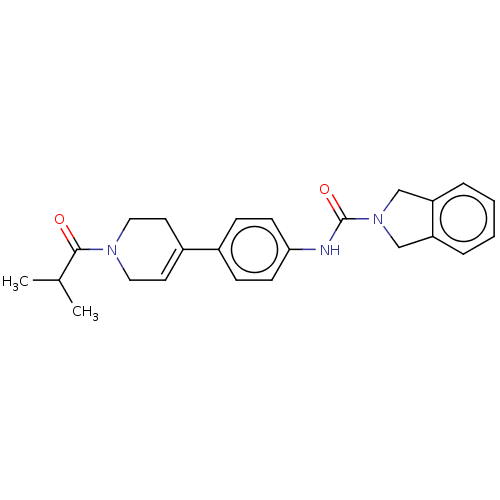 (US9302989, 331) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc. US Patent | Assay Description Probe 1:The assay was carried out in 18 uL low volume plates (Owens Corning) in reaction buffer (50 mM HEPES (NaOH), pH 7.5, 100 mM NaCl, 10 mM MgCl2... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 565 total ) | Next | Last >> |