

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM30185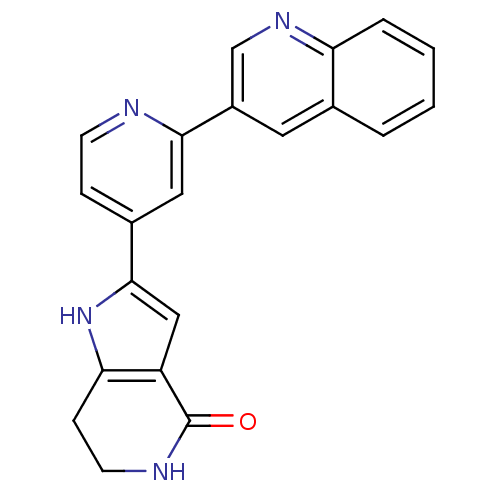 (CHEMBL226403 | Pyrrolopyridine, 16) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM30178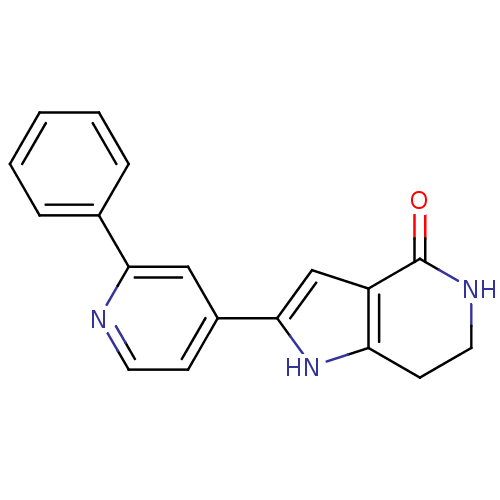 (Pyrrolopyridine, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 3 (Homo sapiens (Human)) | BDBM30185 (CHEMBL226403 | Pyrrolopyridine, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM30192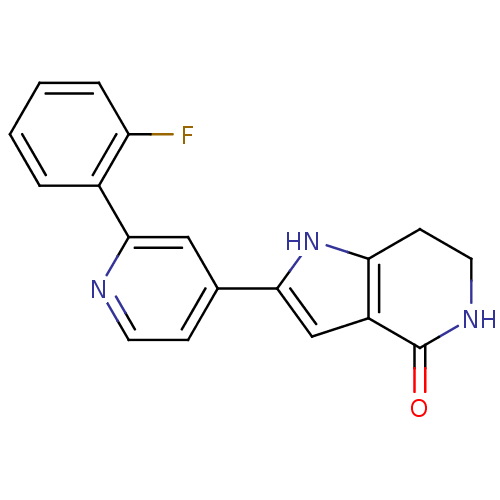 (Pyrrolopyridine, 23) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM27344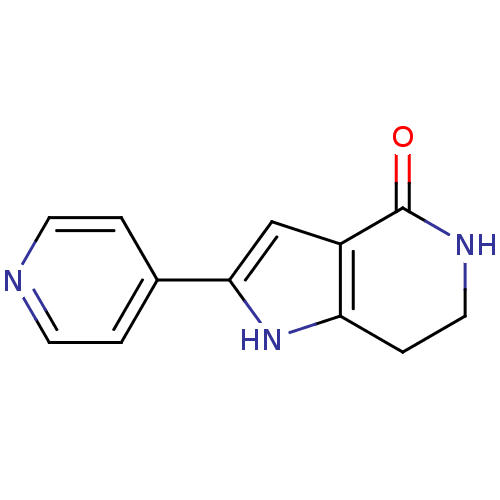 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 3 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| MAP kinase-activated protein kinase 3 (Homo sapiens (Human)) | BDBM30192 (Pyrrolopyridine, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-4 (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM30185 (CHEMBL226403 | Pyrrolopyridine, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM30192 (Pyrrolopyridine, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM30185 (CHEMBL226403 | Pyrrolopyridine, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM30192 (Pyrrolopyridine, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 3 (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.77E+4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Ribosomal protein S6 kinase alpha-4 (Homo sapiens (Human)) | BDBM30192 (Pyrrolopyridine, 23) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-4 (Homo sapiens (Human)) | BDBM30178 (Pyrrolopyridine, 9) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM30185 (CHEMBL226403 | Pyrrolopyridine, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-4 (Homo sapiens (Human)) | BDBM30185 (CHEMBL226403 | Pyrrolopyridine, 16) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM30192 (Pyrrolopyridine, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... | J Med Chem 50: 2647-54 (2007) Article DOI: 10.1021/jm0611004 BindingDB Entry DOI: 10.7270/Q2794313 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||