

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234339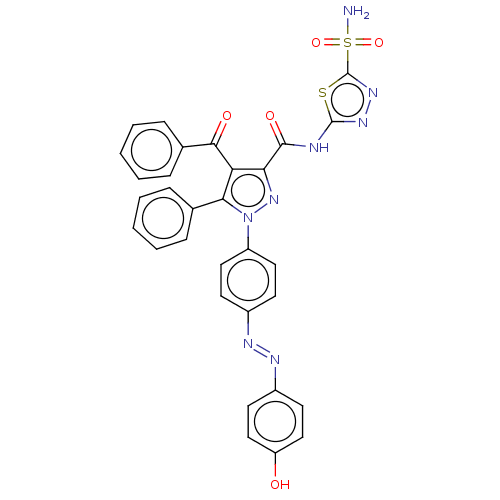 (4-Benzoyl-1-(4-((4-hydroxyphenyl)diazenyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234340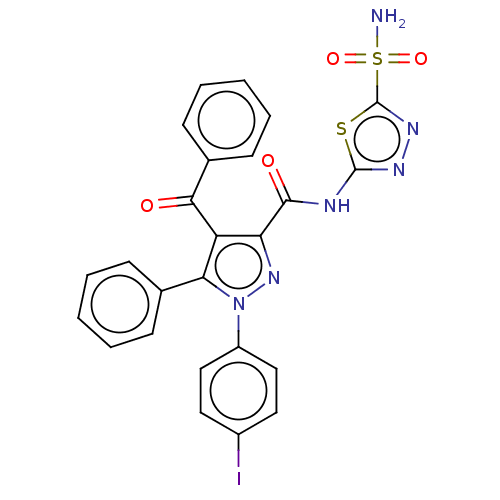 (4-Benzoyl-1-(4-iodophenyl)-5-phenyl-N-(5-sulphamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234346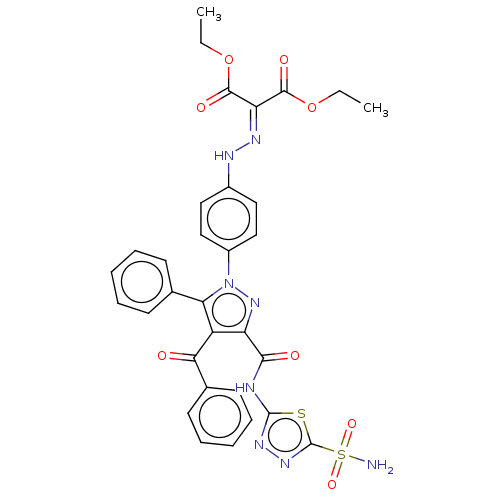 (Diethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234339 (4-Benzoyl-1-(4-((4-hydroxyphenyl)diazenyl)phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234341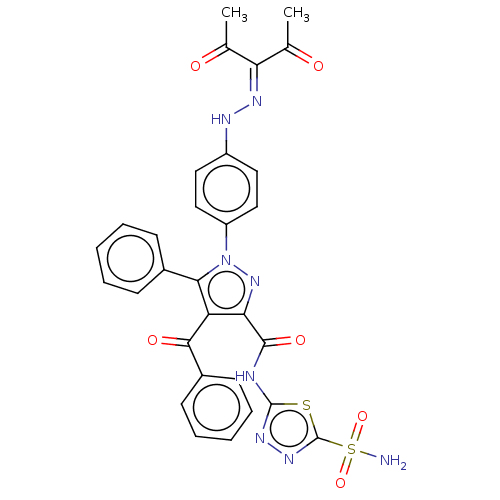 (4-Benzoyl-1-(4-(2-(2,4-dioxopentan-3-ylidene)hydra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234347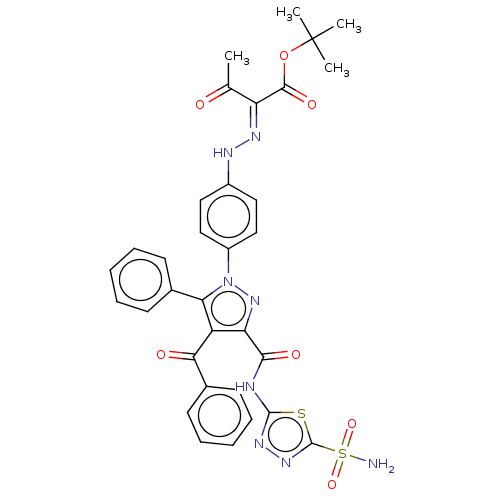 (tert-Butyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulpha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234345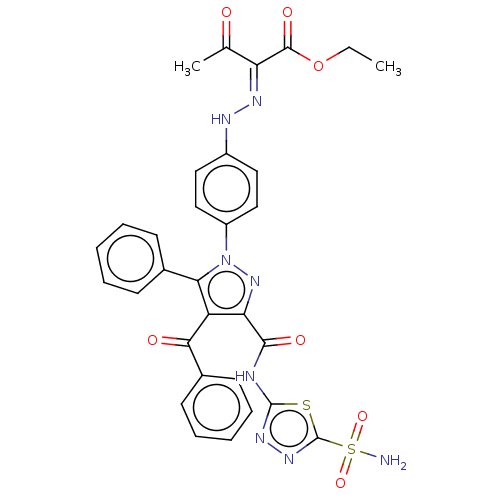 (Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234342 (4-Benzoyl-1-(4-(2-(1,3-dioxo-1-phenylbutan-2-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234346 (Diethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234347 (tert-Butyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulpha...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234340 (4-Benzoyl-1-(4-iodophenyl)-5-phenyl-N-(5-sulphamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234344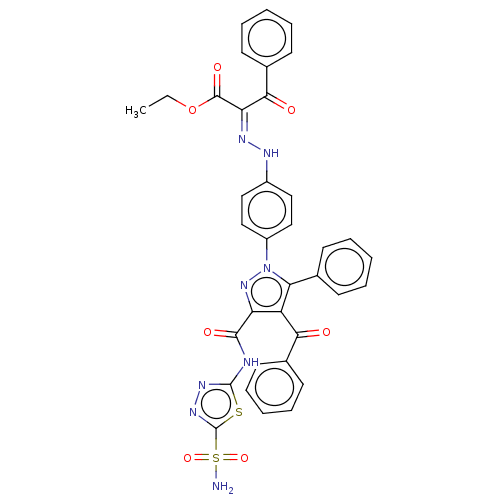 (Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234341 (4-Benzoyl-1-(4-(2-(2,4-dioxopentan-3-ylidene)hydra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234338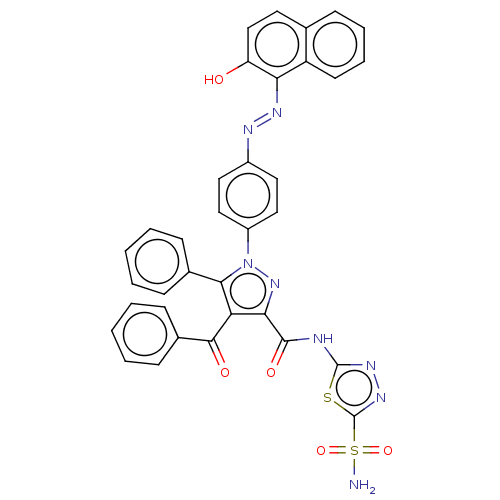 (4-Benzoyl-1-(4-((2-hydroxynaphthalen-1-yl)diazenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 848 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234343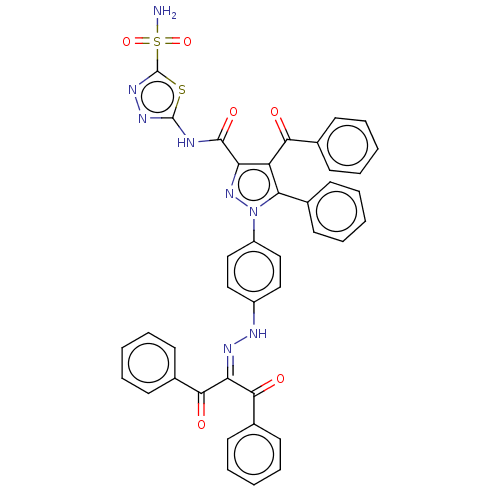 (4-Benzoyl-1-(4-(2-(1,3-dioxo-1,3-diphenylpropan-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234345 (Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234344 (Ethyl 2-(2-(4-(4-benzoyl-5-phenyl-3-(5-sulphamoyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234342 (4-Benzoyl-1-(4-(2-(1,3-dioxo-1-phenylbutan-2-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10868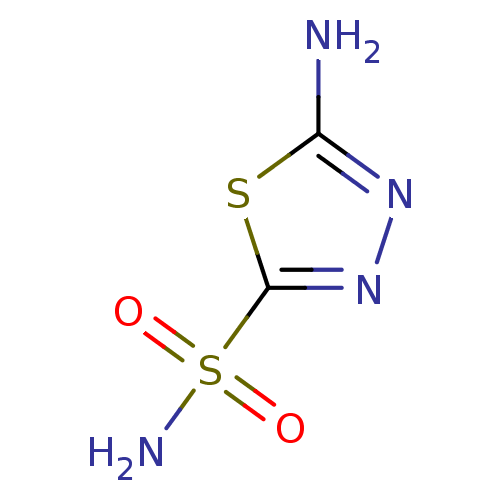 (1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234343 (4-Benzoyl-1-(4-(2-(1,3-dioxo-1,3-diphenylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10868 (1,3,4-Thiadiazole-2-sulfonamide, 6 | 1,3,4-thiadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM234348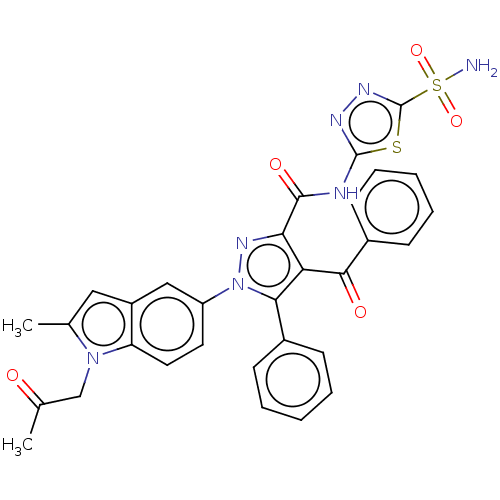 (4-Benzoyl-1-(2-methyl-1-(2-oxopropyl)-1H-indol-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234338 (4-Benzoyl-1-(4-((2-hydroxynaphthalen-1-yl)diazenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM234348 (4-Benzoyl-1-(2-methyl-1-(2-oxopropyl)-1H-indol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dumlupinar University | Assay Description The Ki values were determined as described in the literature [Landolfi et al., J. Pharmacol. Toxicol. Methods, 38:169-172; Bülbül et al., J. Enzyme I... | J Enzyme Inhib Med Chem 26: 231-7 (2011) Article DOI: 10.3109/14756366.2010.491795 BindingDB Entry DOI: 10.7270/Q2DZ0754 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||