

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50017698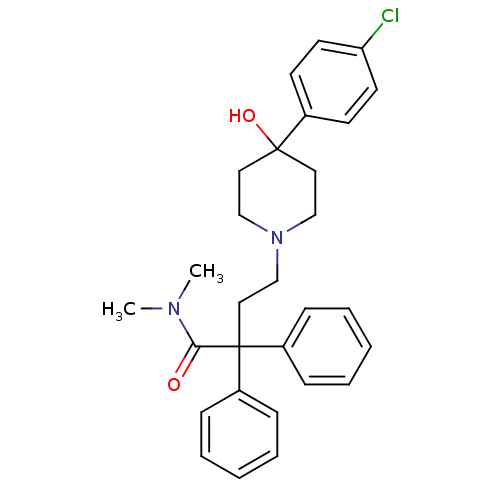 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154040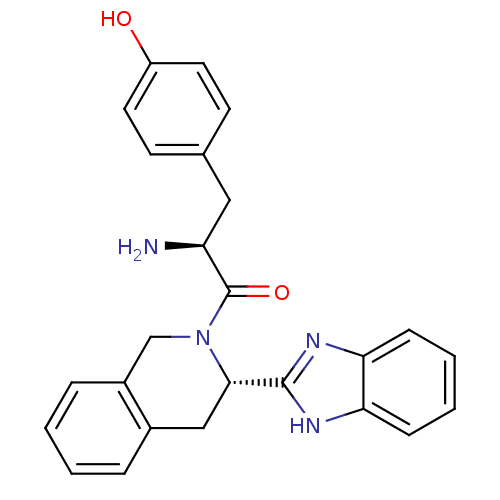 (2-Amino-1-[3-(1H-benzoimidazol-2-yl)-3,4-dihydro-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154039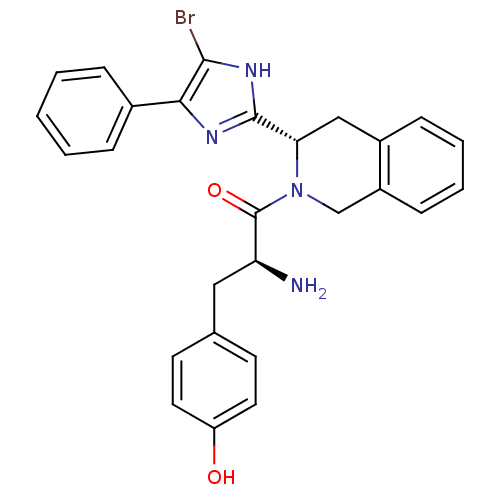 (2-Amino-1-[3-(5-bromo-4-phenyl-1H-imidazol-2-yl)-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154037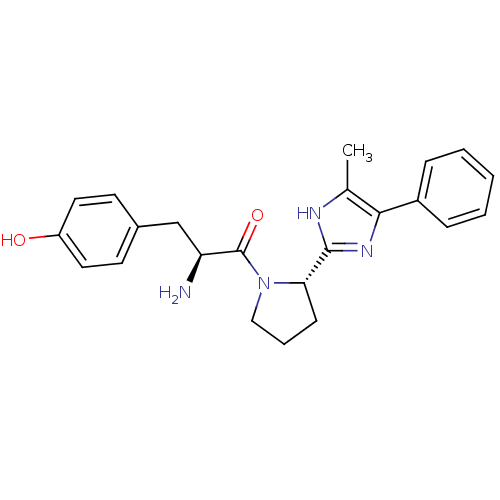 (2-Amino-3-(4-hydroxy-phenyl)-1-[2-(5-methyl-4-phen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154041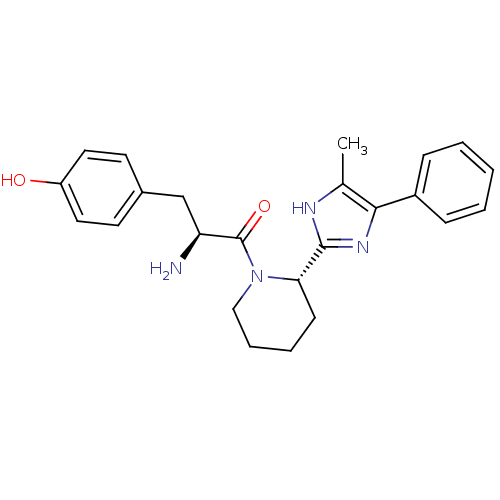 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154036 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-3-(4-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154042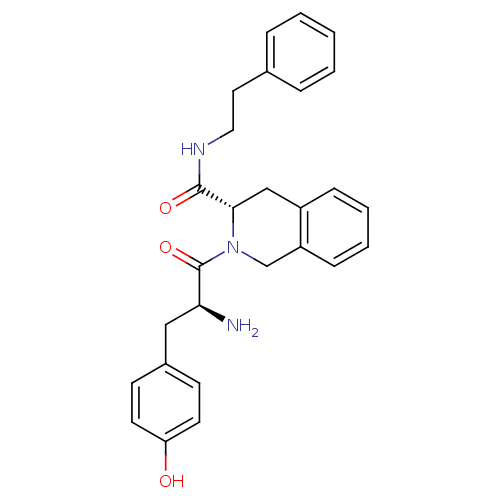 (2-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-1,2,3,4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154035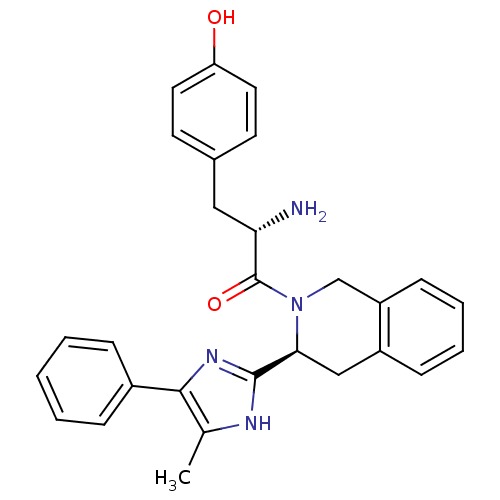 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-3-(5-methyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||