

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073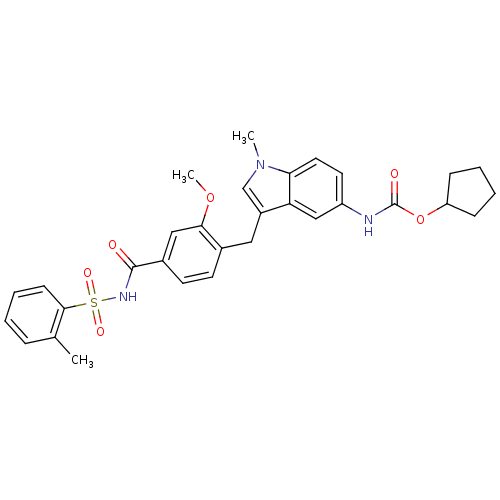 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM189379 (5-O-ethyl 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088492 (Bumecaine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103612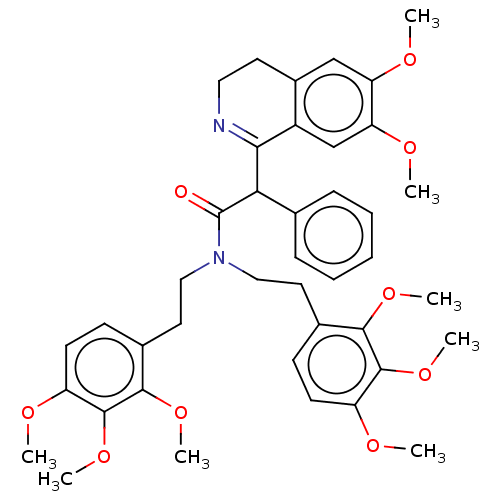 (CHEMBL2356116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50301375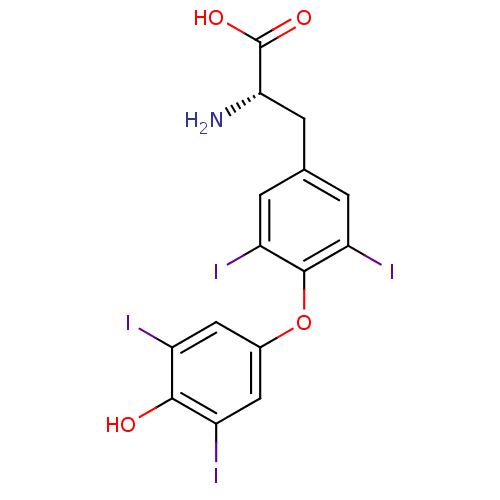 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103615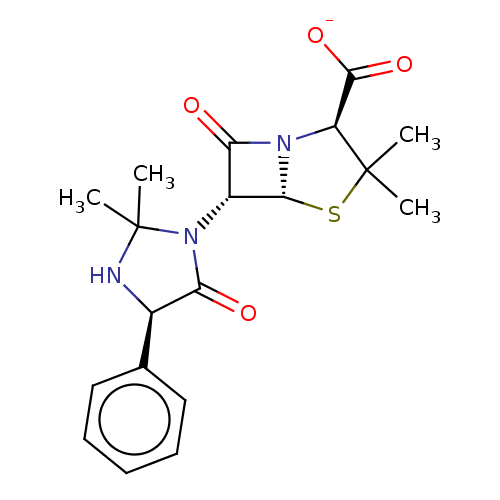 (CHEBI:34789 | Hetacillin Potassium | Versapen-K) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103614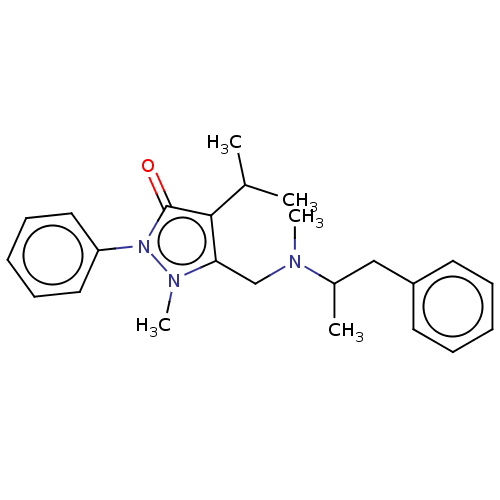 (Famprofazone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103619 (CHEMBL1411951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM24567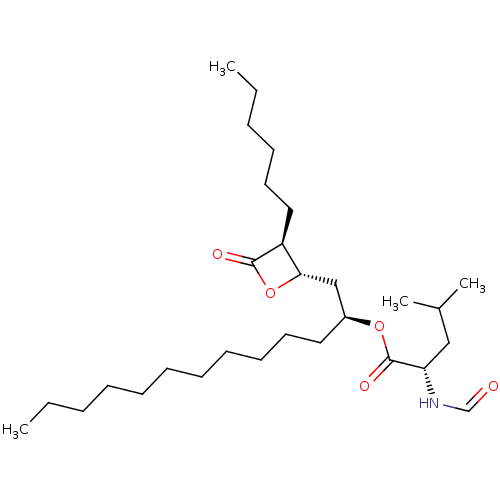 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088493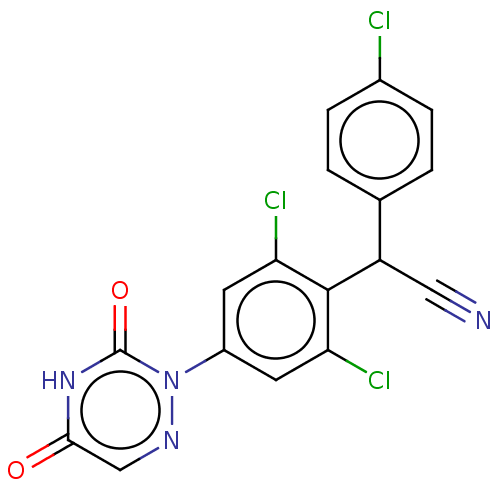 (Diclazuril | R-64433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103618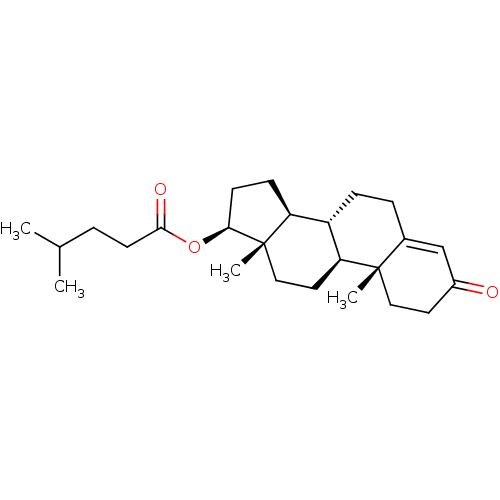 (CHEBI:35001 | CHEMBL3189011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM32073 ((7R,11S)-11-methyl-7,15,17-tris(oxidanyl)-12-oxabi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50008984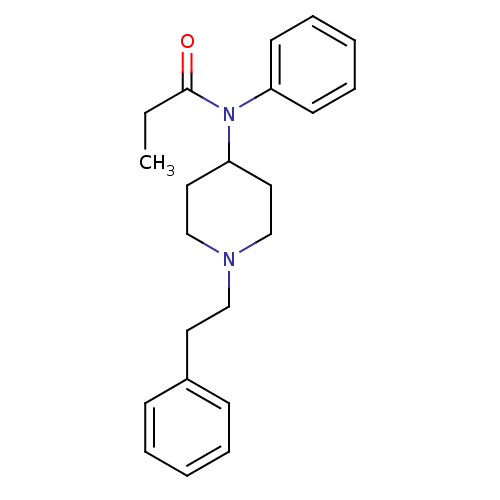 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50061101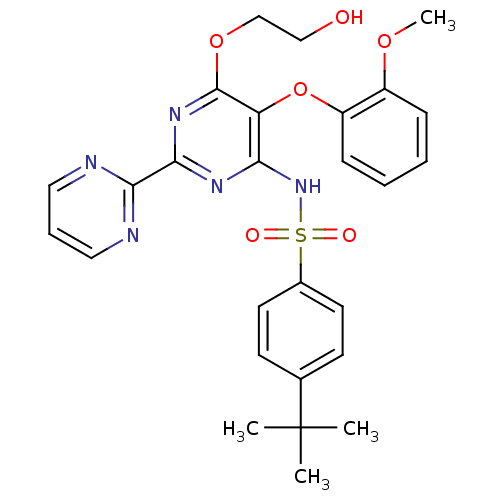 (4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103632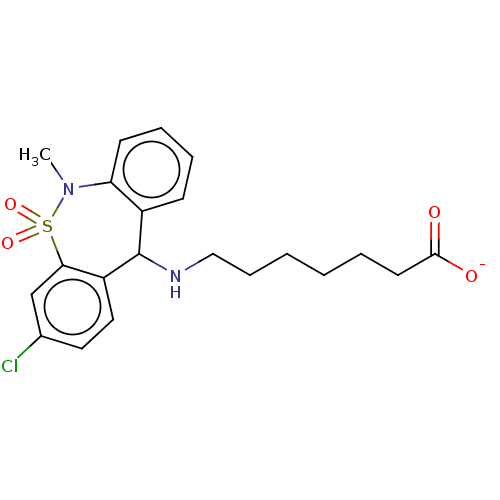 (CHEMBL2361572) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50017716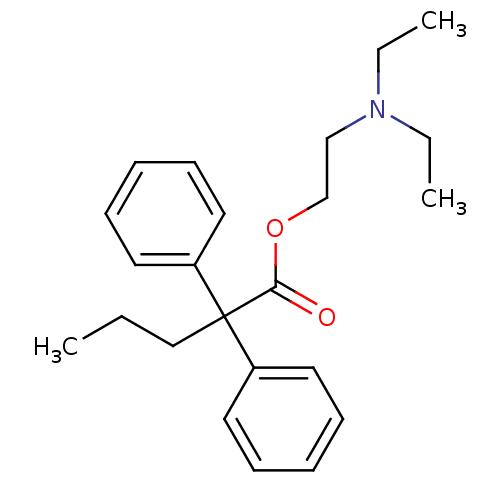 (2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103623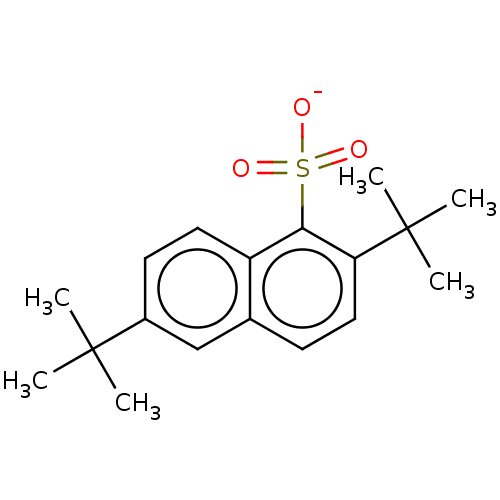 (L-1633 | Sodium Dibunate) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM79589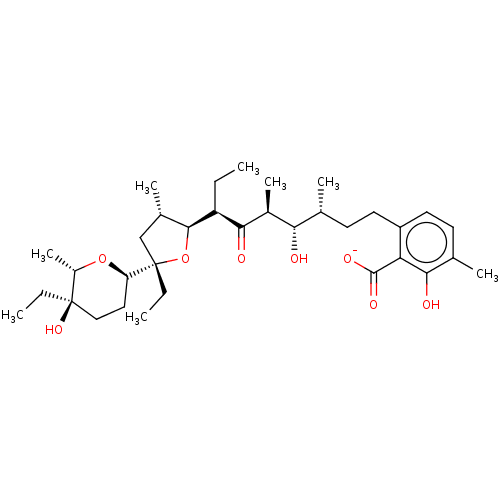 (LASALOCID A SODIUM | MLS001304052 | SMR000538906 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103636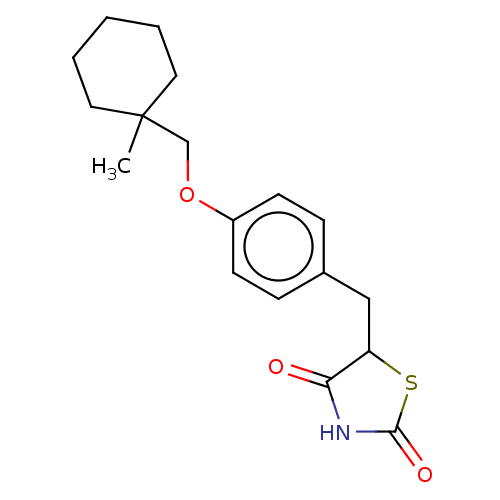 (ADD-3878 | CHEBI:64227 | Ciglitazone | U-63287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103627 (CHEBI:31550 | GNF-Pf-193 | Patupilone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50106731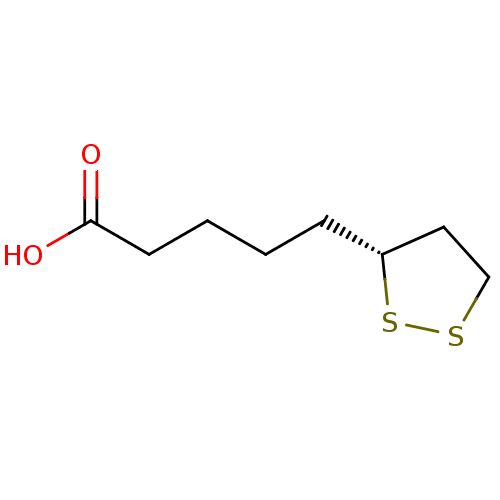 ((+)-alpha-Lipoic acid | (R)-(+)-lipoic acid | (R)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM59083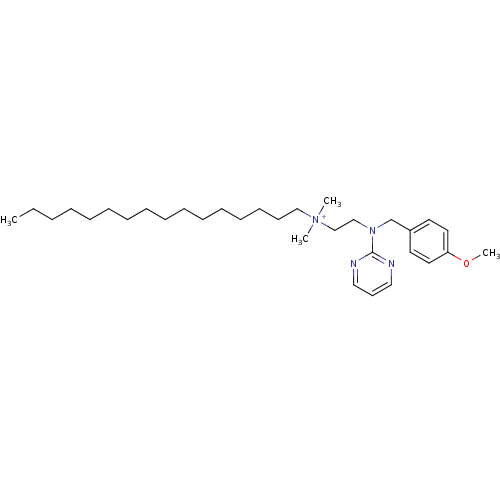 (MLS002154065 | SMR001233380 | THONZONIUM BROMIDE |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103629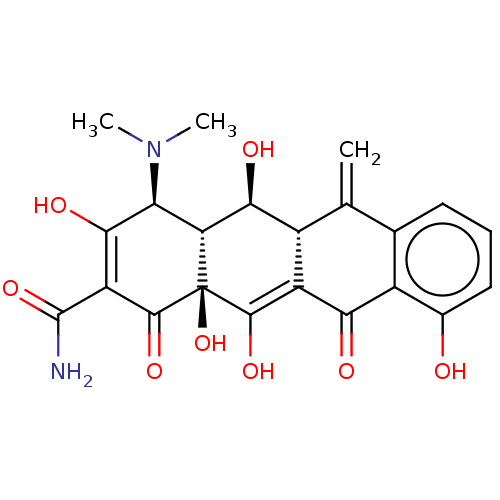 (CHEBI:6806 | METHACYCLINE HYDROCHLORIDE | Methacyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM36880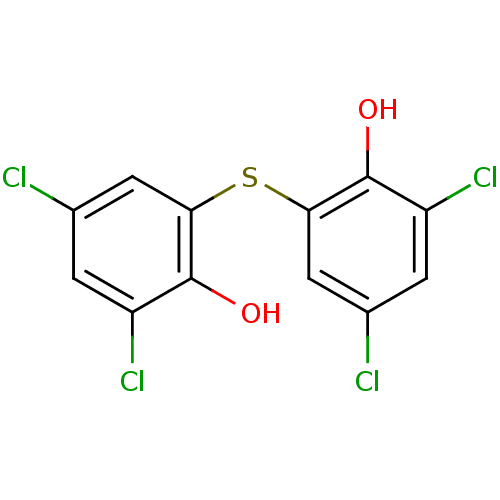 (2,2'-THIOBIS(4,6-DICHLOROPHENOL) | 2,4-dichlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50370232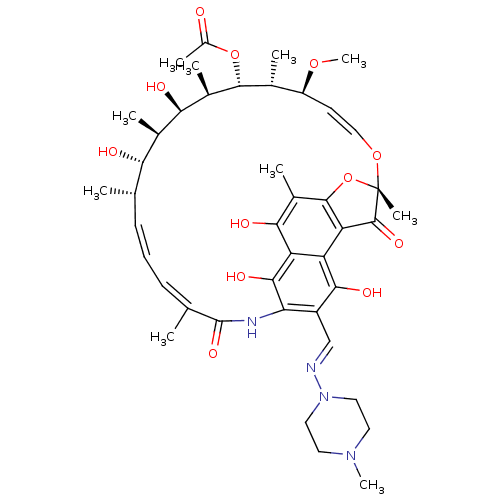 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103607 (Surital | Thiamylal Sodium) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103621 (A-61589 | AA-861 | CHEBI:2340 | Docebenone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50030448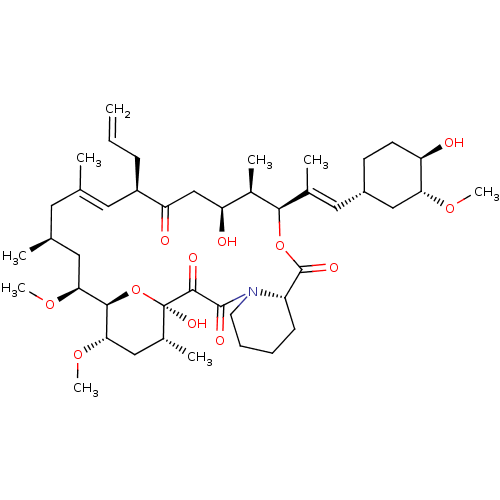 (8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN | CHEMBL269732 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM36609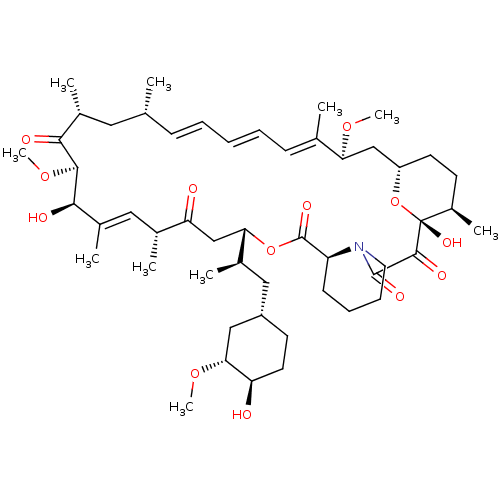 (Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 890 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103613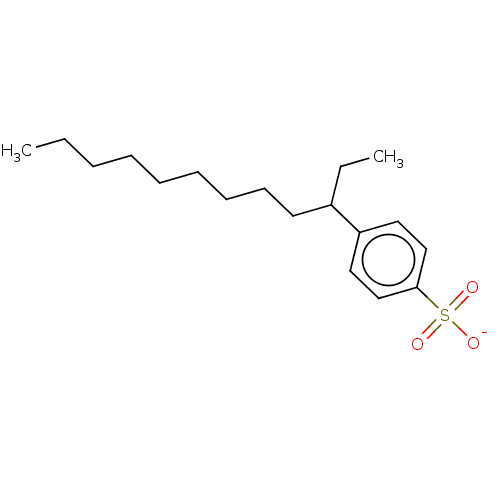 (CHEMBL1720065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM76863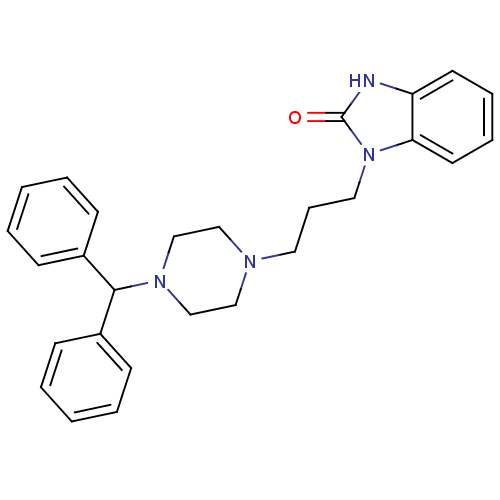 (3-[3-(4-benzhydrylpiperazin-1-yl)propyl]-1H-benzim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50004823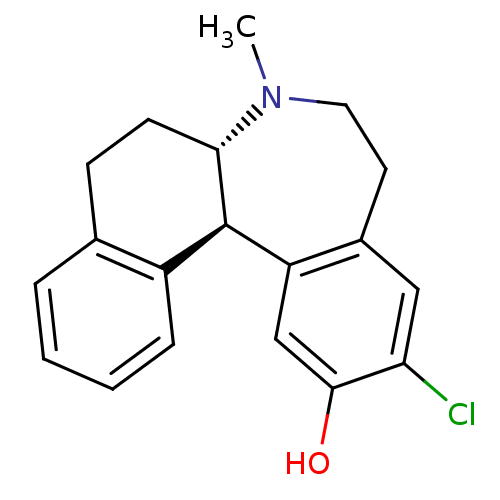 ((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103625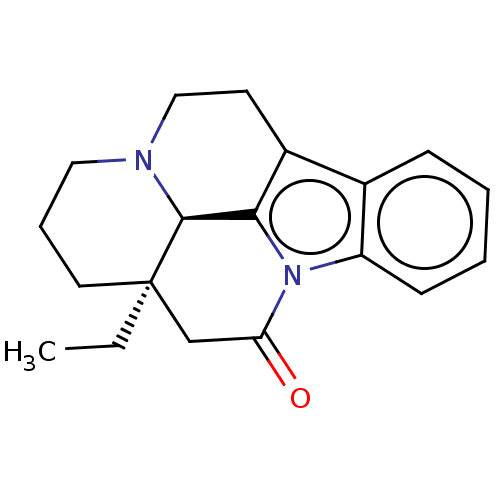 (CHEMBL1318553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103624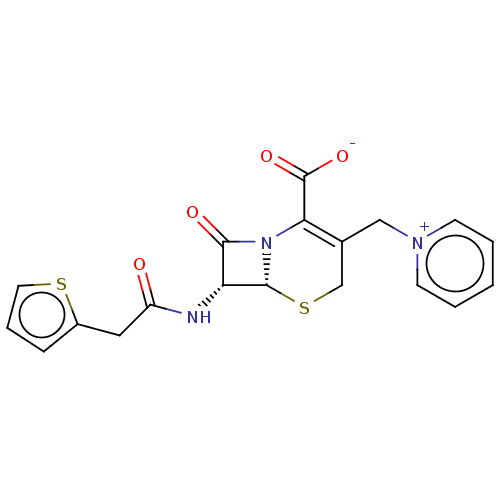 (40602 | CHEBI:3537 | Cefaloridine | Cephaloridine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50020192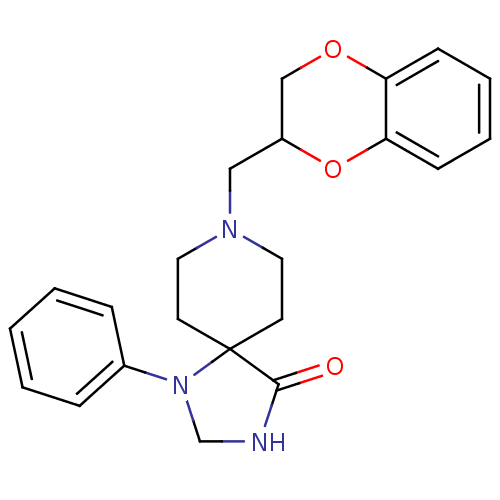 (8-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-1-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50194601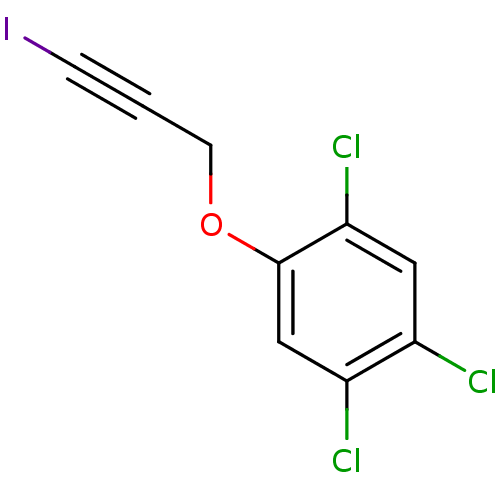 (1,2,4-Trichloro-5-(3-iodo-prop-2-ynyloxy)-benzene ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50240347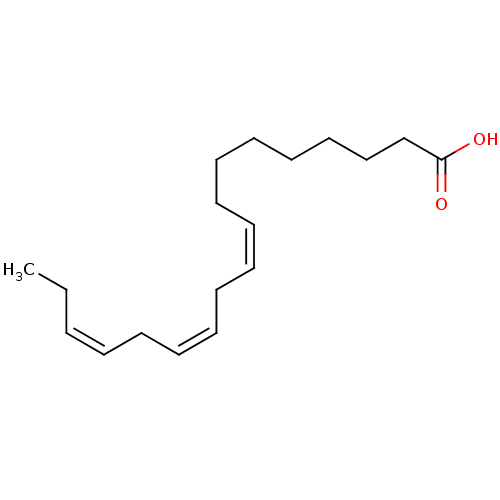 ((9,12,15)-linolenic acid | (9Z,12Z,15Z)-octadeca-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM60994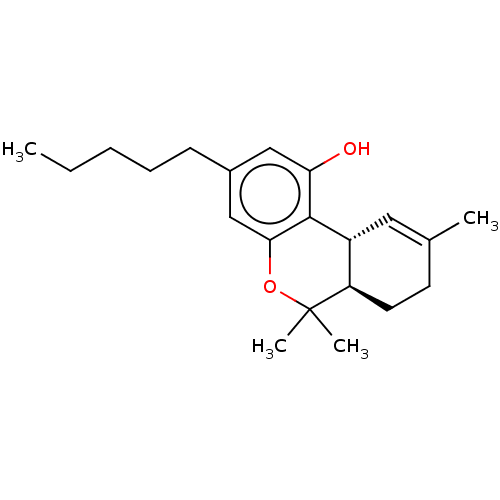 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088383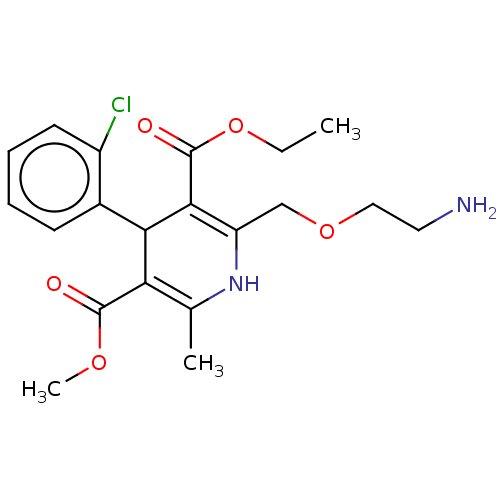 (Amlodipine | CHEBI:2668 | Norvasc) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50336799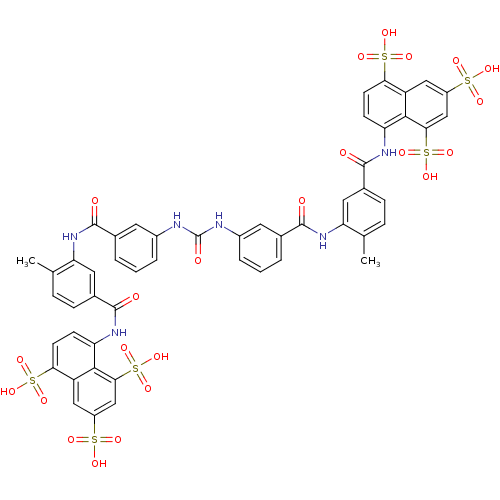 (5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103763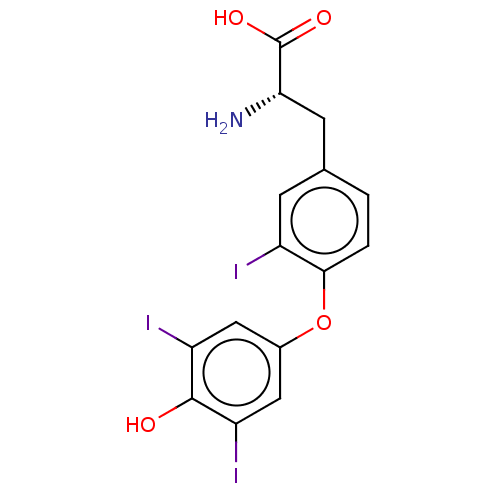 (CHEBI:11684 | REVERSE TRIIODOTHYRONINE | Reverse T...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103630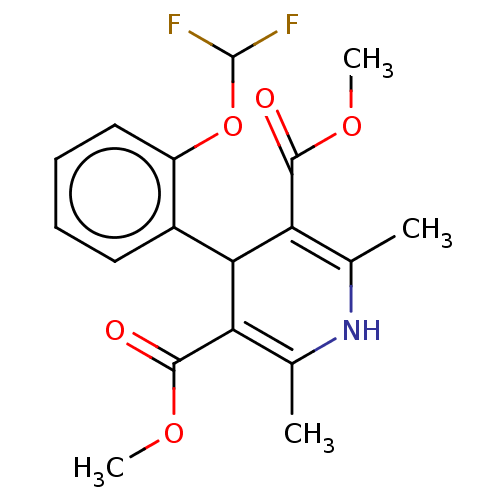 (Riodipine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50014872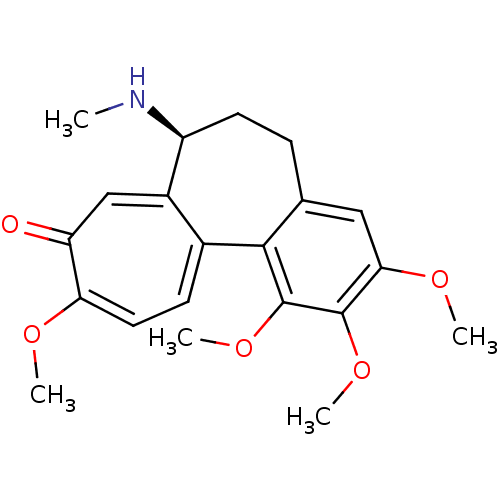 ((-)-demecolcine | (S)-1,2,3,10-tetramethoxy-7-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50406398 (CHEMBL40883 | MDL-72422) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103634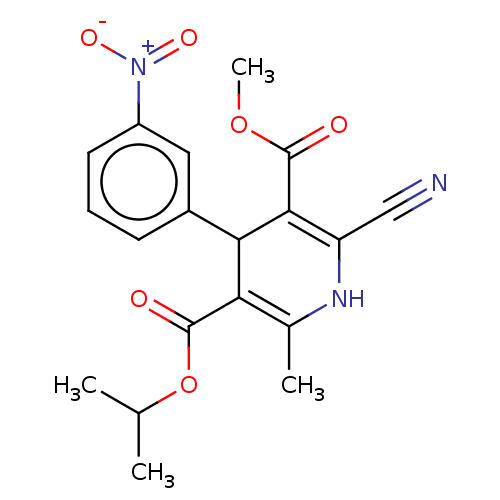 (CL-287389 | FK-235 | Nilvadipine | Nivadipine | SK...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 890 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50010261 ((3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-6,10,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50030477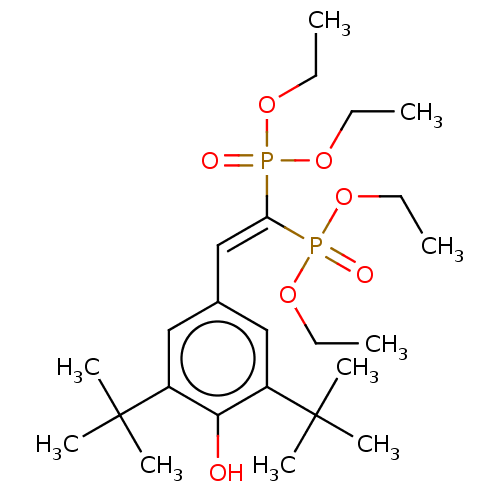 (CHEBI:77317 | CHEMBL458767 | SR-12813) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103617 (CHEMBL451483) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50101963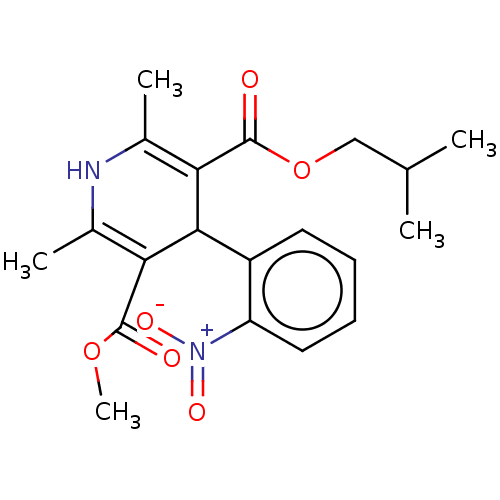 (BAY-K-5552 | CHEBI:76917 | Nisoldipine | Sular) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |