

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148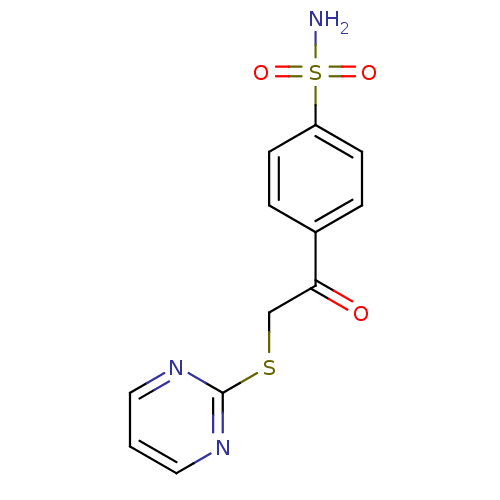 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 9.20E+5 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144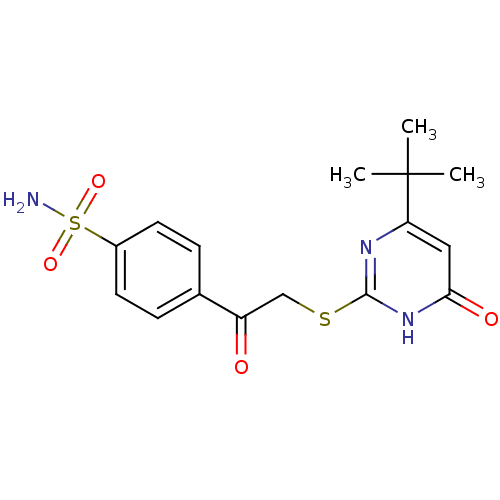 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 440 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 6 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.00E+5 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859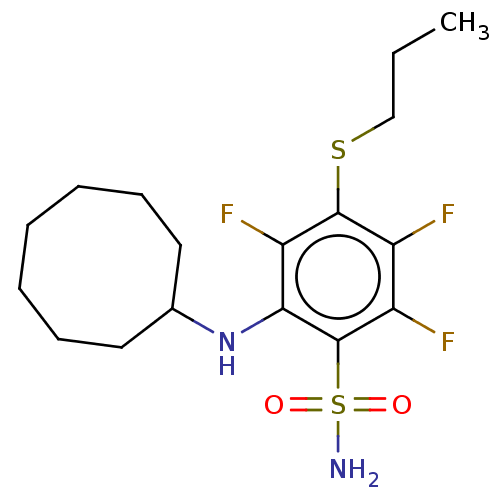 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 5.80E+4 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.290 | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858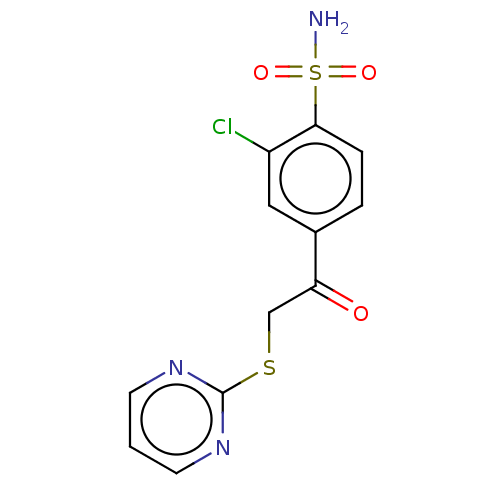 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.430 | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.150 | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00790 | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 330 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 6 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 6 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 360 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 6 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 140 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 6 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 330 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 6 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 940 | n/a | n/a | n/a | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 6 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.40E+5 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 56 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 7 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00720 | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 80 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 7 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 46 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 7 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 46 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 7 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 160 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 7 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 260 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from saturation signal at pH 7 by surface pla... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.140 | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.300 | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.140 | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163859 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+5 | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.10E+6 | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80E+6 | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.30E+6 | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as association rate constant at pH 7 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 62 | n/a | n/a | n/a | 7.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA9 catalytic domain assessed as dissociation constant calculated from kinetic parameters at pH 7 by surface pl... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM85658 (EZA4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 34 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10886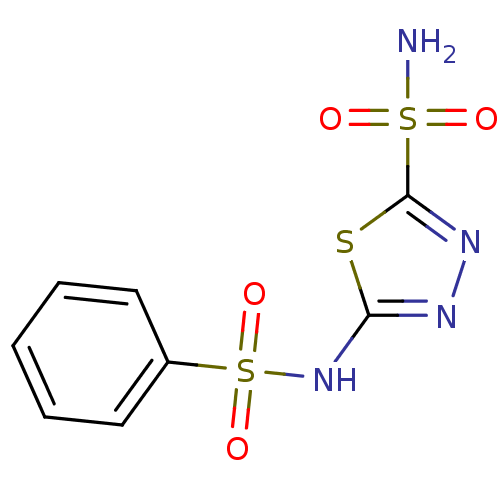 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 45 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM85659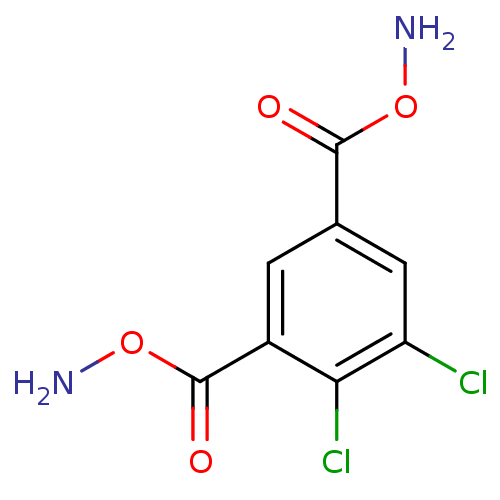 (DCP5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103914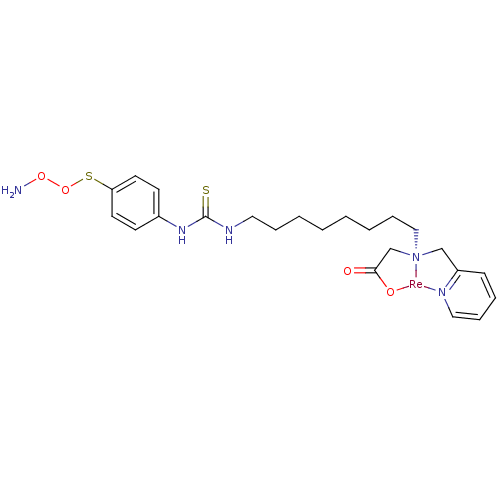 (US8562945, 249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103908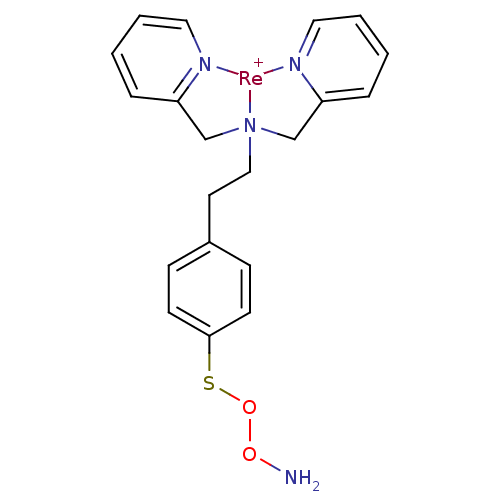 (JNK3 inhibitor 5 | US8562945, 241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103913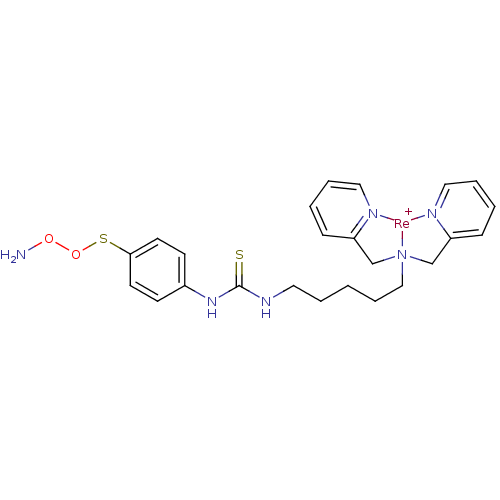 (US8562945, 248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103912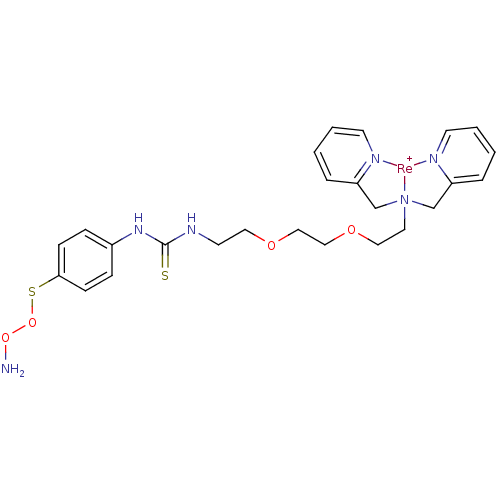 (US8562945, 247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 305 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103911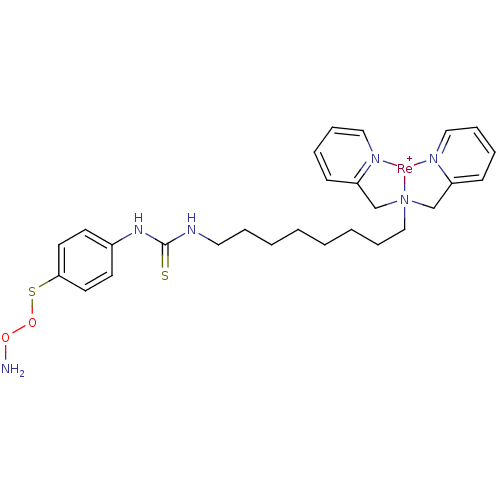 (JNK3 inhibitor 8 | US8562945, 246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103910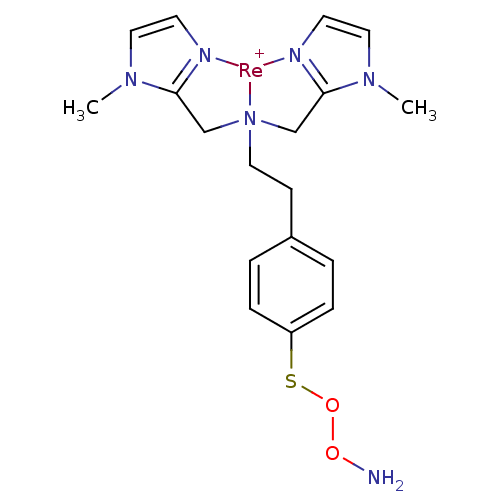 (JNK3 inhibitor 7 | US8562945, 243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 564 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103909 (JNK3 inhibitor 6 | US8562945, 242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103915 (US8562945, 250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM82103 (Investigational agent, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 4.90 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... | Chem Biol Drug Des 74: 317-21 (2009) Article DOI: 10.1111/j.1747-0285.2009.00857.x BindingDB Entry DOI: 10.7270/Q2833QHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10888 (1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.10 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... | Chem Biol Drug Des 74: 317-21 (2009) Article DOI: 10.1111/j.1747-0285.2009.00857.x BindingDB Entry DOI: 10.7270/Q2833QHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM150250 (US8980932, 6b (DH309)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stichting Maastricht Radiation Oncology “Maastro-Clinic”; Université Montpellier 2 Sciences et Techniques US Patent | Assay Description The inhibition constants (K) the compounds for four CA isozymes, CA I, II, IX and XII were determined. An Applied Photophysics (Oxford, UK) stopped-f... | US Patent US8980932 (2015) BindingDB Entry DOI: 10.7270/Q2GX499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM150249 (US8980932, 6a (DH307)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stichting Maastricht Radiation Oncology “Maastro-Clinic”; Université Montpellier 2 Sciences et Techniques US Patent | Assay Description The inhibition constants (K) the compounds for four CA isozymes, CA I, II, IX and XII were determined. An Applied Photophysics (Oxford, UK) stopped-f... | US Patent US8980932 (2015) BindingDB Entry DOI: 10.7270/Q2GX499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM150254 (US8980932, 4d (DH302)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stichting Maastricht Radiation Oncology “Maastro-Clinic”; Université Montpellier 2 Sciences et Techniques US Patent | Assay Description The inhibition constants (K) the compounds for four CA isozymes, CA I, II, IX and XII were determined. An Applied Photophysics (Oxford, UK) stopped-f... | US Patent US8980932 (2015) BindingDB Entry DOI: 10.7270/Q2GX499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM82104 (Investigational agent, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.80 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 20 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description Inhibition assay using carbonic anhydrases with an SX.18MV-R, a stopped-flow instrument from Applied Photophysics was used for assaying the CA cataly... | Chem Biol Drug Des 74: 317-21 (2009) Article DOI: 10.1111/j.1747-0285.2009.00857.x BindingDB Entry DOI: 10.7270/Q2833QHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM150252 (US8980932, 6d (DH308)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Stichting Maastricht Radiation Oncology “Maastro-Clinic”; Université Montpellier 2 Sciences et Techniques US Patent | Assay Description The inhibition constants (K) the compounds for four CA isozymes, CA I, II, IX and XII were determined. An Applied Photophysics (Oxford, UK) stopped-f... | US Patent US8980932 (2015) BindingDB Entry DOI: 10.7270/Q2GX499J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |