

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.50E+4 | >-27.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 16: 3755-60 (2006) Article DOI: 10.1016/j.bmcl.2006.04.044 BindingDB Entry DOI: 10.7270/Q2319T41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31459 (CHEMBL224485 | substituted biphenyl derivative, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31488 (substituted biphenyl derivative, 36ab) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31461 (substituted biphenyl derivative, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31462 (substituted biphenyl derivative, 36b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31463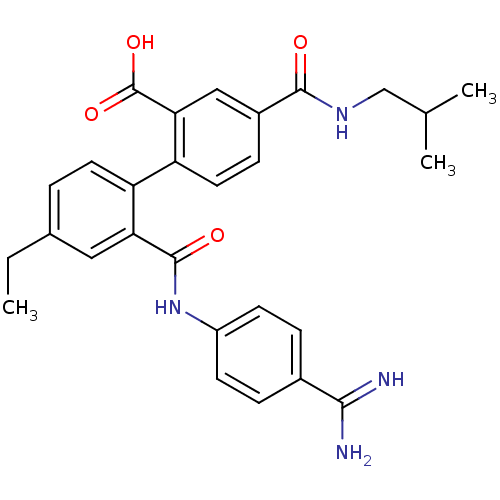 (substituted biphenyl derivative, 36c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31464 (substituted biphenyl derivative, 36d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31465 (substituted biphenyl derivative, 36e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31466 (substituted biphenyl derivative, 36f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31467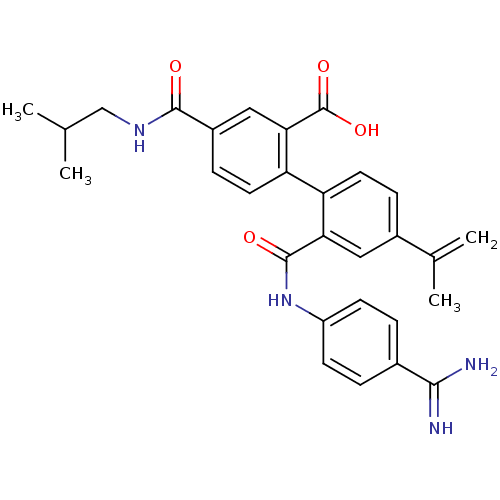 (substituted biphenyl derivative, 36g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31468 (substituted biphenyl derivative, 36h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31469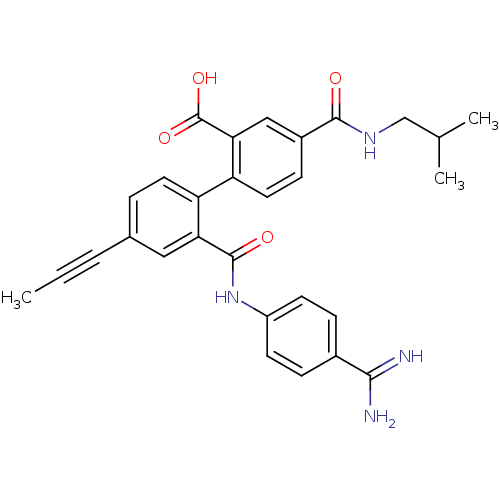 (substituted biphenyl derivative, 36i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31470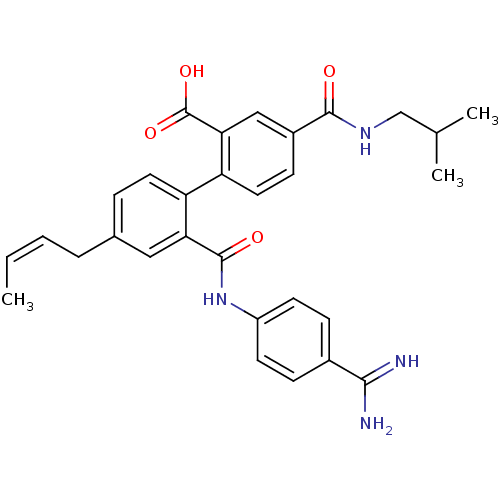 (substituted biphenyl derivative, 36j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31471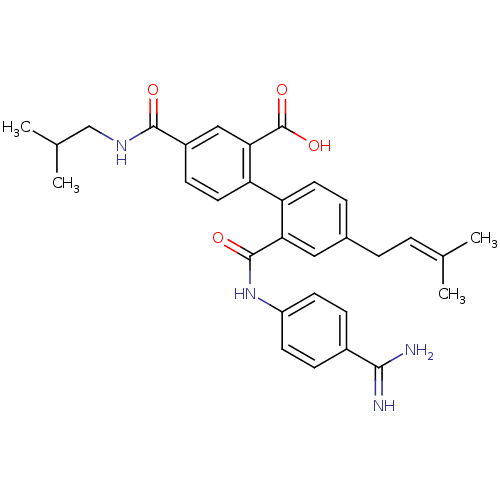 (substituted biphenyl derivative, 36k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31472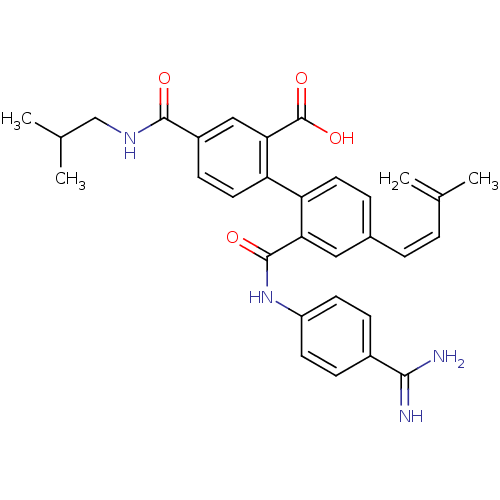 (substituted biphenyl derivative, 36l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31473 (substituted biphenyl derivative, 36m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31474 (substituted biphenyl derivative, 36n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31475 (substituted biphenyl derivative, 36o) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31476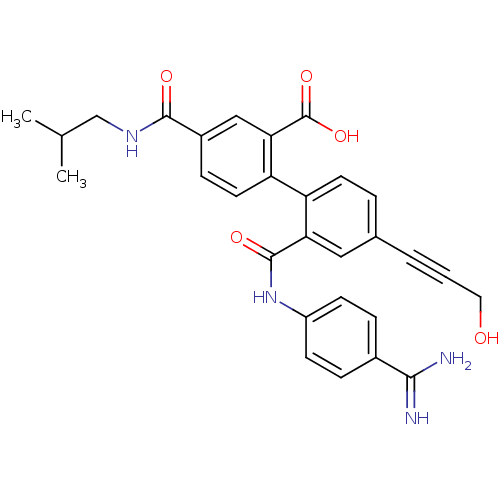 (substituted biphenyl derivative, 36p) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31477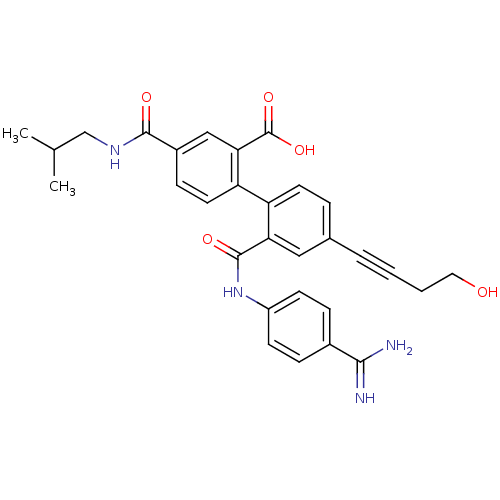 (substituted biphenyl derivative, 36q) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31478 (substituted biphenyl derivative, 36r) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 926 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31479 (substituted biphenyl derivative, 36s) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31480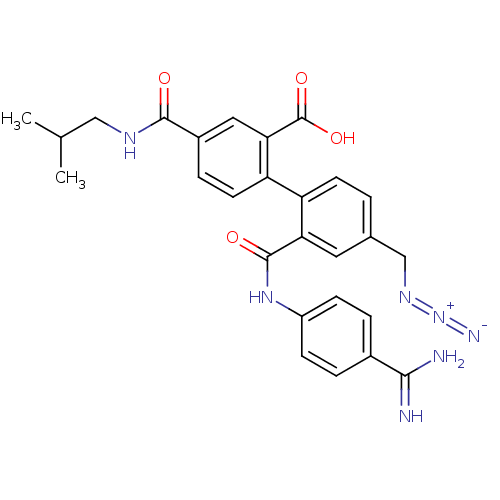 (substituted biphenyl derivative, 36t) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31481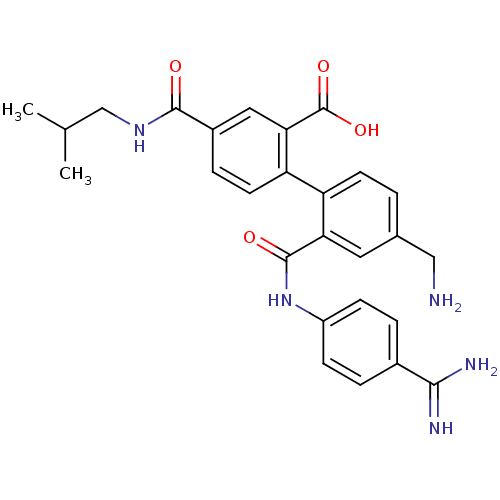 (substituted biphenyl derivative, 36u) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31482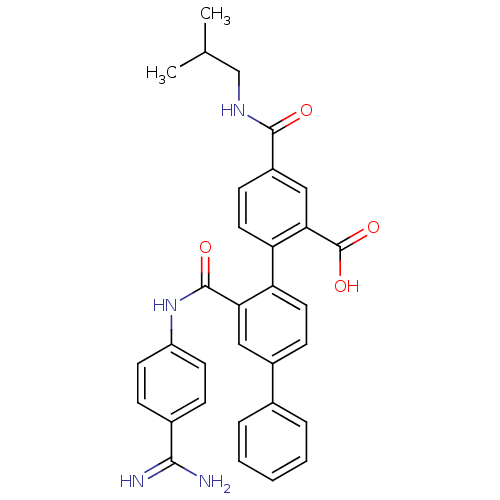 (substituted biphenyl derivative, 36v) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31483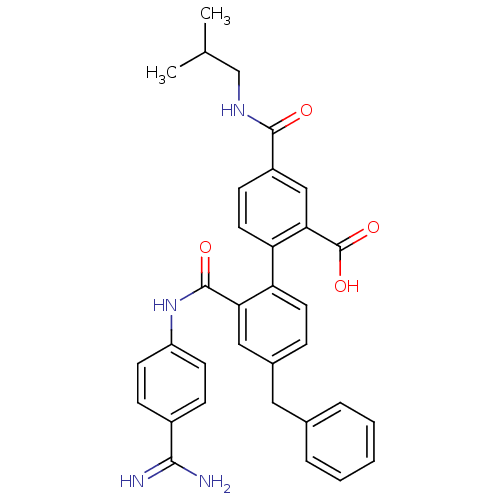 (substituted biphenyl derivative, 36w) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31484 (substituted biphenyl derivative, 36x) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31485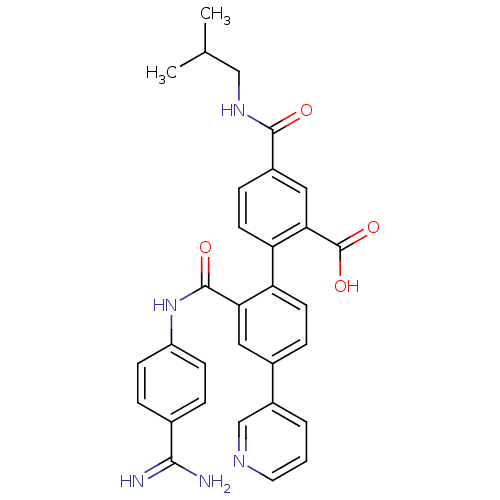 (substituted biphenyl derivative, 36y) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 546 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31486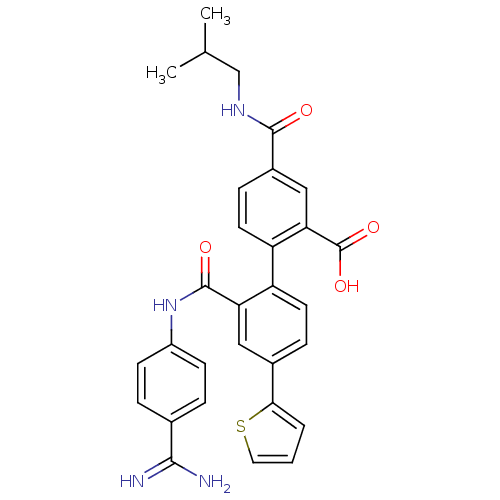 (substituted biphenyl derivative, 36z) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31487 (substituted biphenyl derivative, 36aa) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31460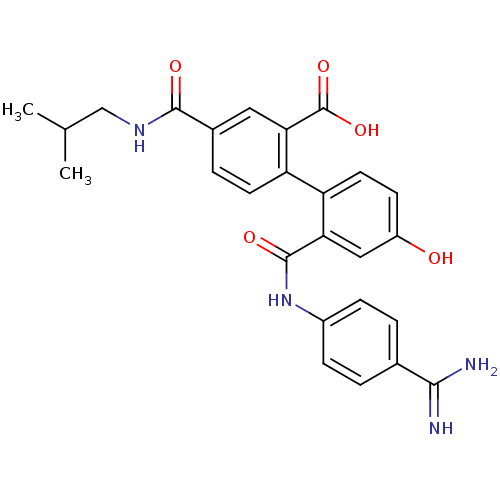 (substituted biphenyl derivative, 36a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.2 | 23 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13778 (2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14898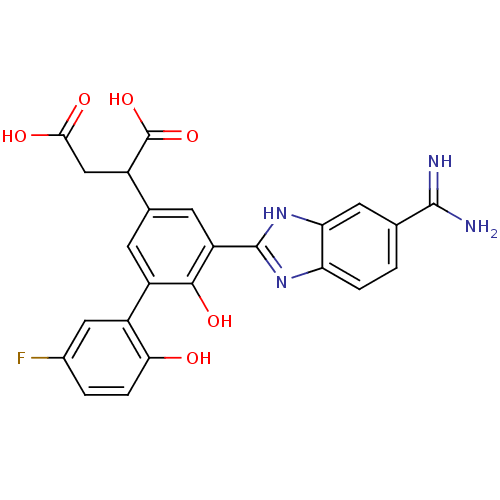 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14899 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14900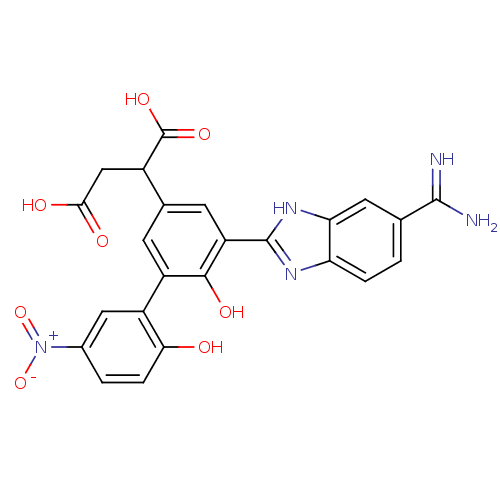 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14904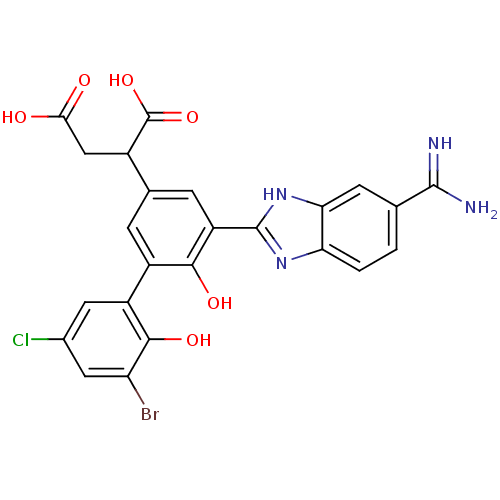 (2-[3-(3-bromo-5-chloro-2-hydroxyphenyl)-5-(5-carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14901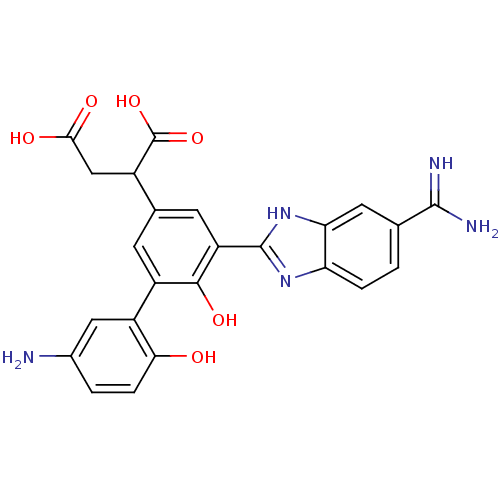 (2-[3-(5-amino-2-hydroxyphenyl)-5-(5-carbamimidoyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14902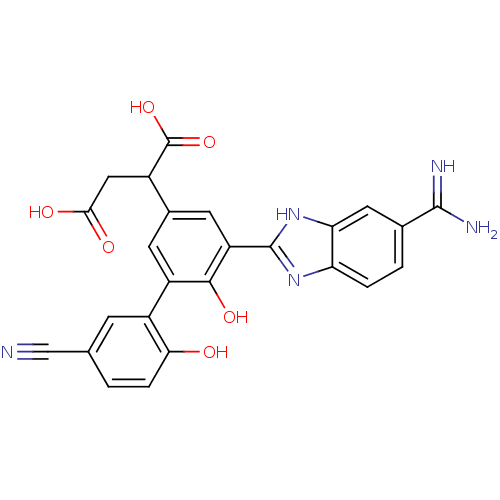 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14903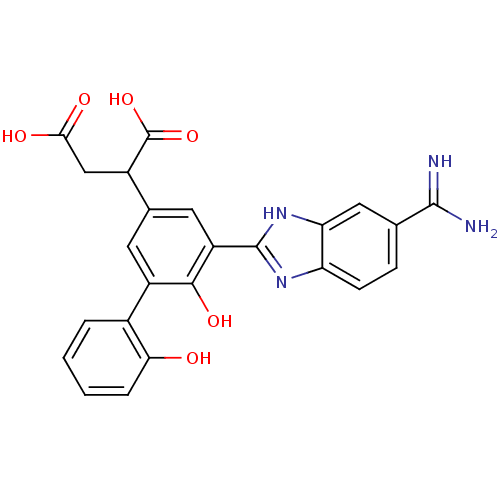 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14863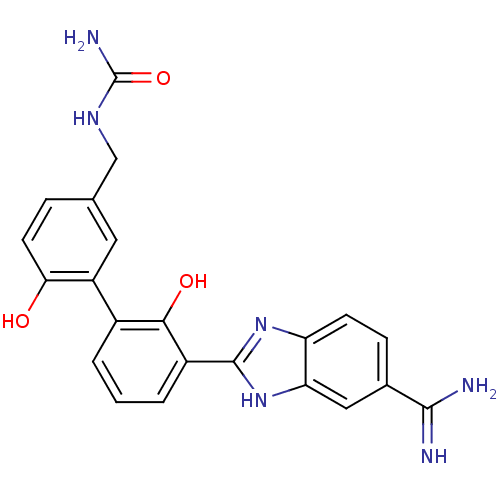 (({3-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 3197-200 (2006) Article DOI: 10.1016/j.bmcl.2006.03.049 BindingDB Entry DOI: 10.7270/Q20P0X9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14905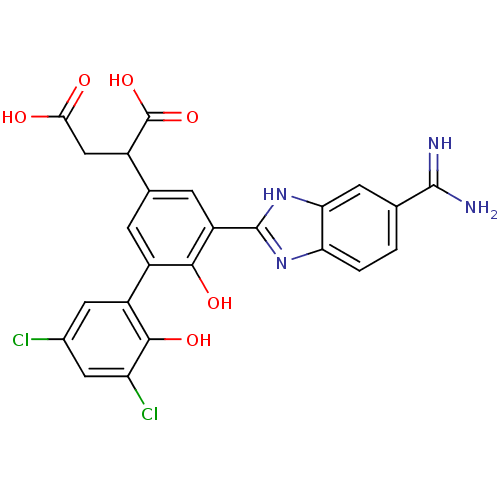 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13776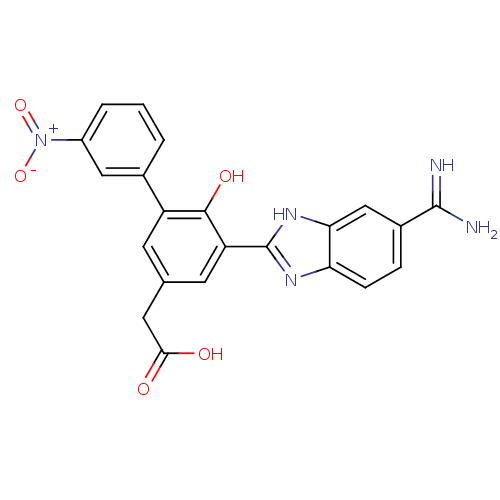 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13786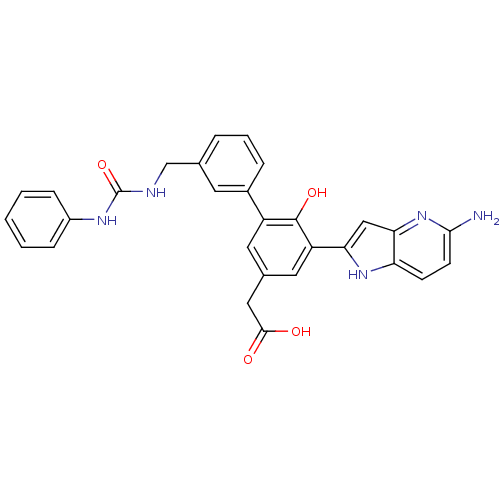 (2-(3-{5-amino-1H-pyrrolo[3,2-b]pyridin-2-yl}-4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 2270-3 (2006) Article DOI: 10.1016/j.bmcl.2006.01.017 BindingDB Entry DOI: 10.7270/Q2RF5S8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14907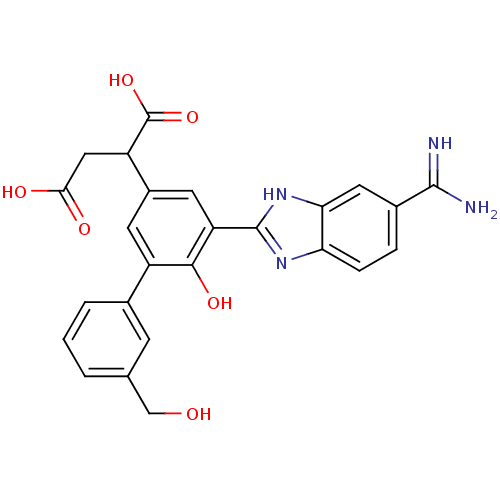 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14909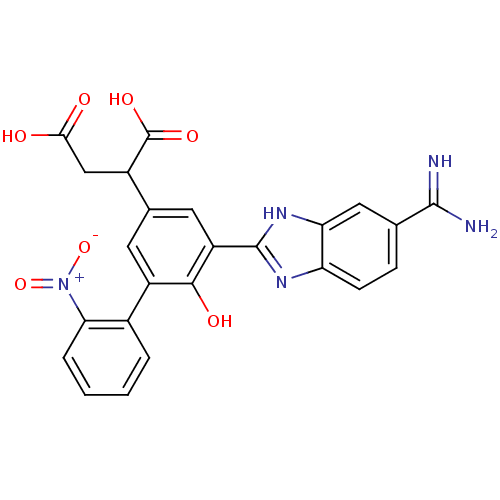 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14908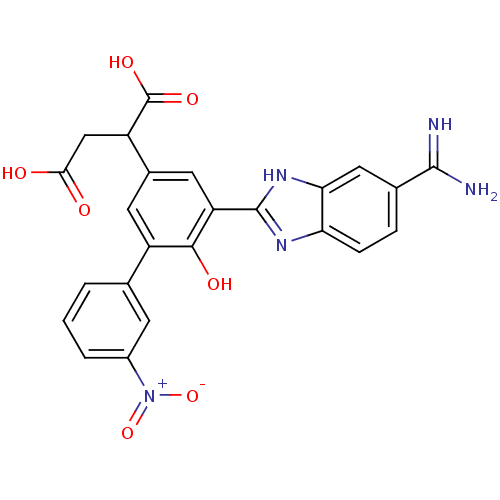 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14906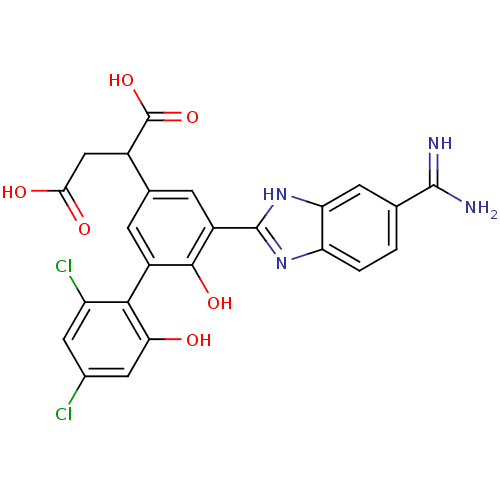 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14910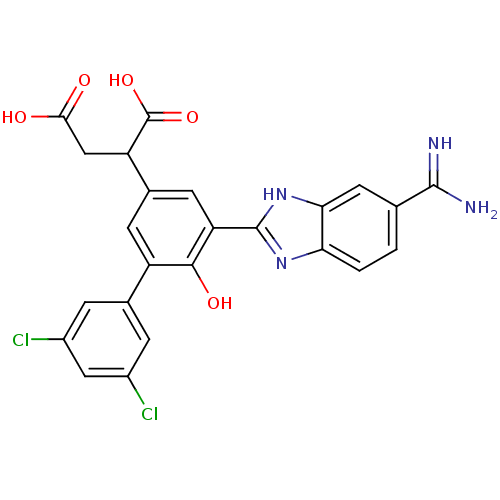 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14911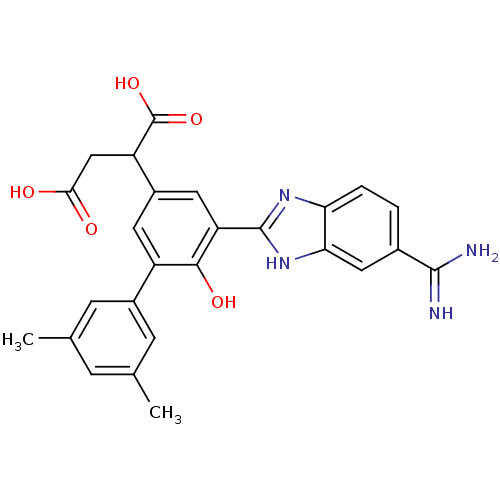 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM14912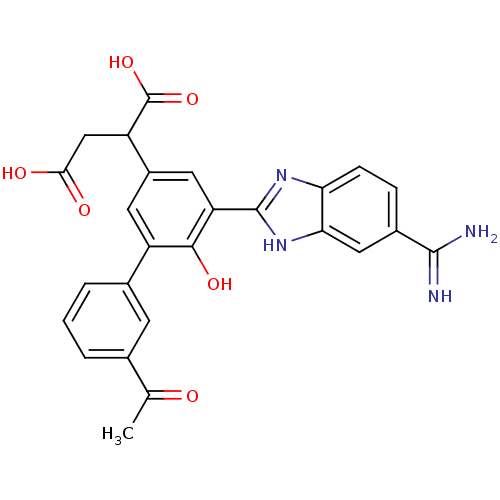 (2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 16: 1596-600 (2006) Article DOI: 10.1016/j.bmcl.2005.12.040 BindingDB Entry DOI: 10.7270/Q2VX0DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |