

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247414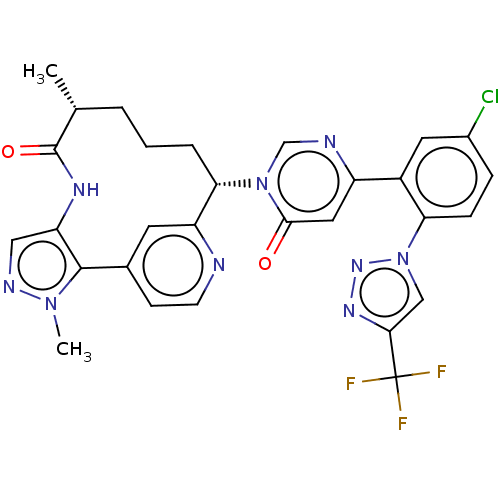 (US10336754, Example 368 | US11053247, Example 368 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247417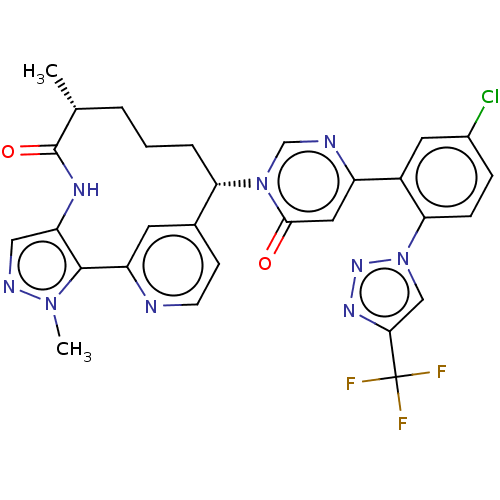 (US10336754, Example 371 | US11053247, Example 371 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247416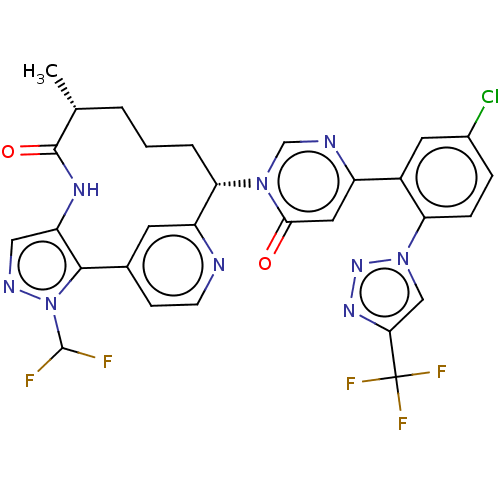 (US10336754, Example 369 | US11053247, Example 369 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247411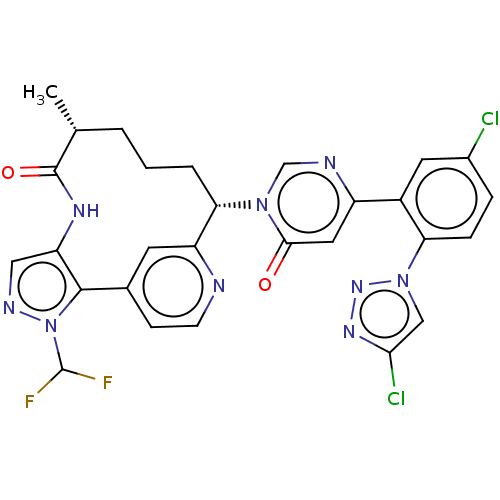 (US10336754, Example 353 | US11053247, Example 353 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247414 (US10336754, Example 368 | US11053247, Example 368 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247417 (US10336754, Example 371 | US11053247, Example 371 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247419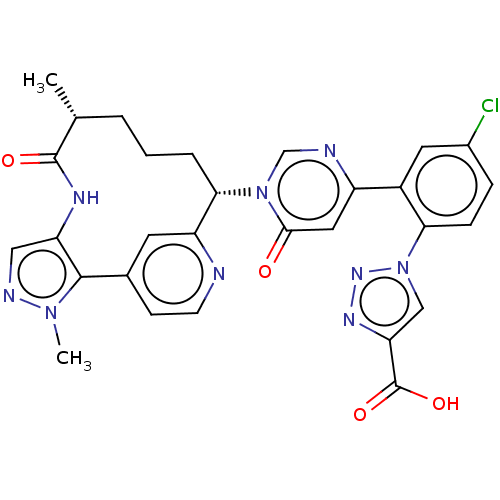 (US10336754, Example 373 | US11053247, Example 373 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247413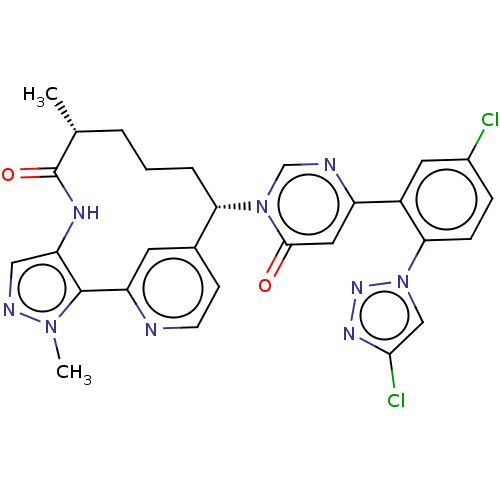 (US10336754, Example 363 | US11053247, Example 363 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247413 (US10336754, Example 363 | US11053247, Example 363 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247418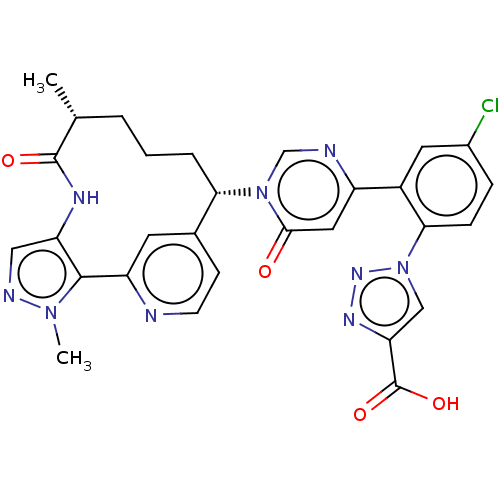 (US10336754, Example 372 | US11053247, Example 372 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | -57.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289804 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM247412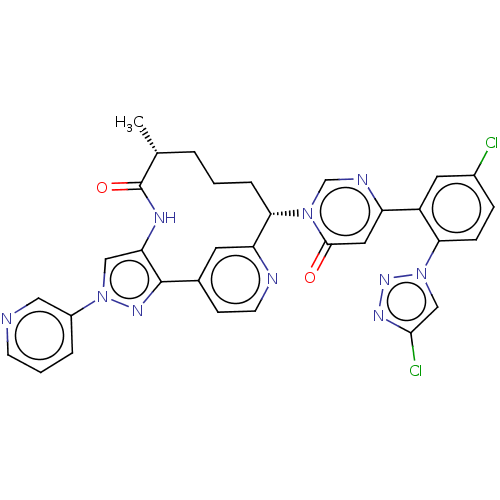 (US10336754, Example 354 | US11053247, Example 354 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9453018 (2016) BindingDB Entry DOI: 10.7270/Q25X27VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289851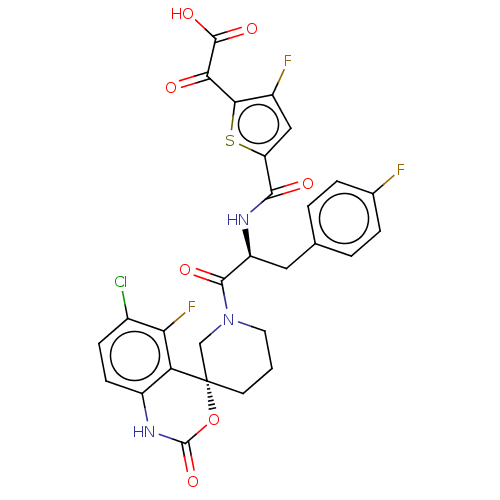 (2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807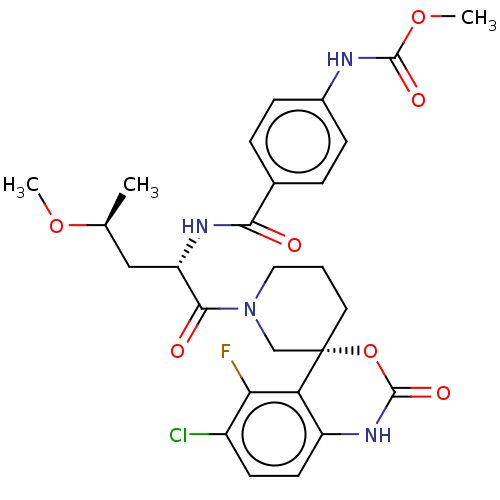 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289844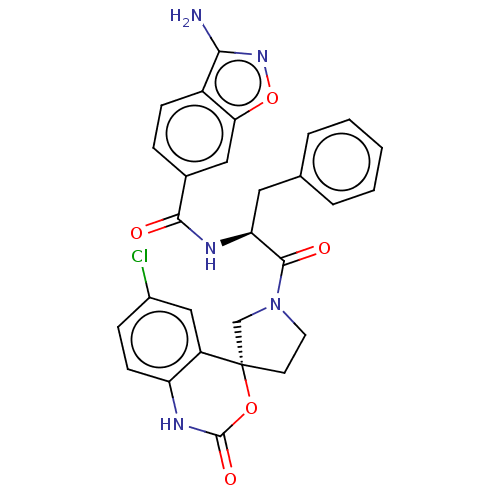 (3-amino-N—((S)-1-(R)-6-chloro-2-oxo-1,2-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.52 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289795 (Methyl 2-amino-3-(5-fluoropyridin-2-yl)propanoate ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.82 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285852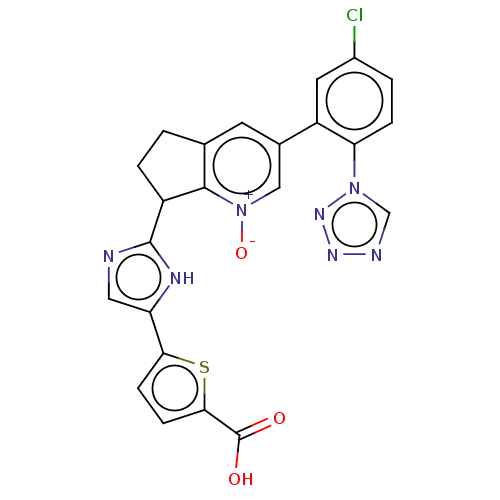 (5-(2-{3-[5-chloro-2- (1H-tetrazol-1- yl)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10081617 (2018) BindingDB Entry DOI: 10.7270/Q2CR5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289853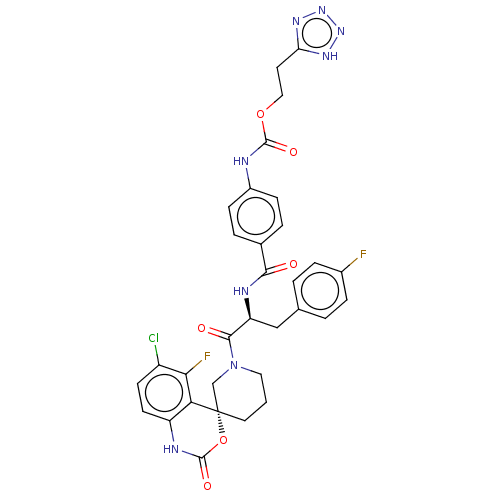 (2-(1H-tetrazol-5-yl)ethyl (4-(((S)-1-((R)-6-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.22 | -48.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805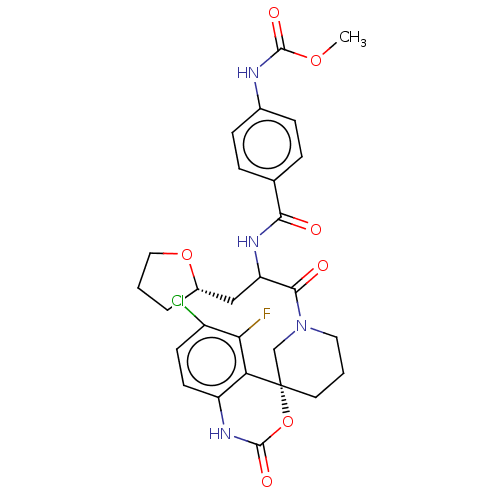 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.21 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM131023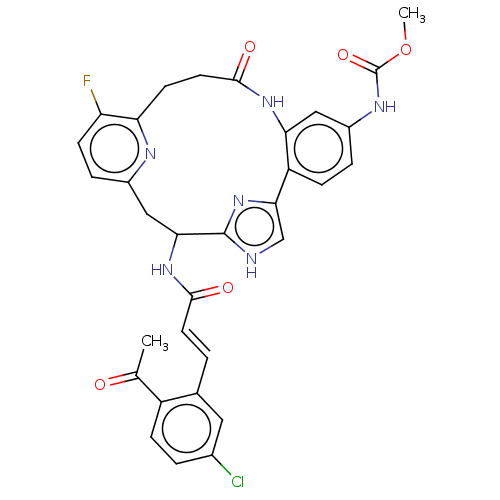 (US8828983, 140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US8828983 (2014) BindingDB Entry DOI: 10.7270/Q2FB51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US8828983 (2014) BindingDB Entry DOI: 10.7270/Q2FB51NX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286021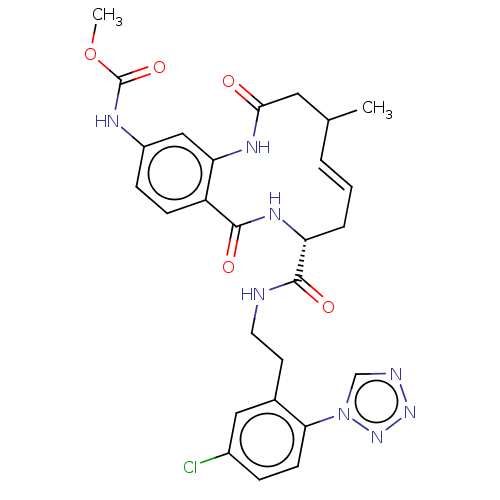 ((E)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286012 ((E)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286009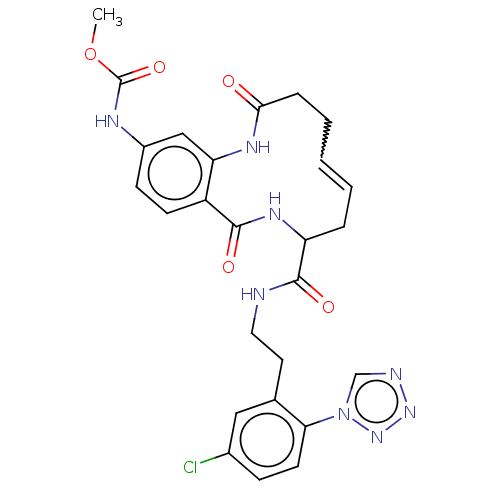 ((E/Z)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM222993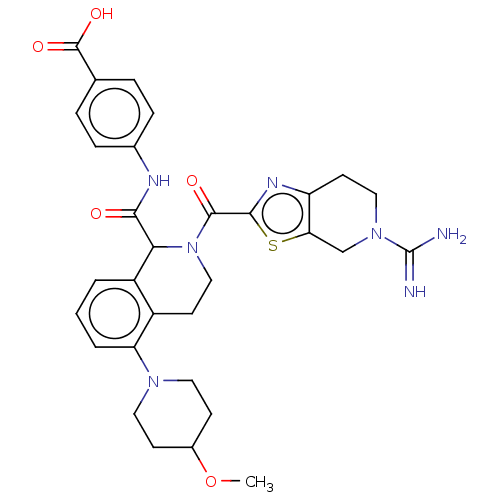 (US9315519, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9315519 (2016) BindingDB Entry DOI: 10.7270/Q2BC3XFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM222995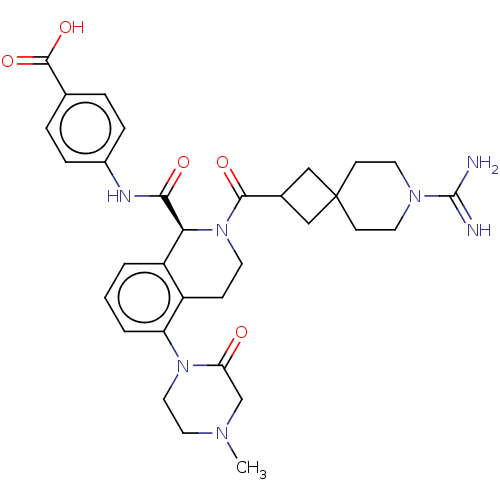 (US9315519, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9315519 (2016) BindingDB Entry DOI: 10.7270/Q2BC3XFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161055 (US9108951, 10 | US9394276, 10 | US9725435, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161058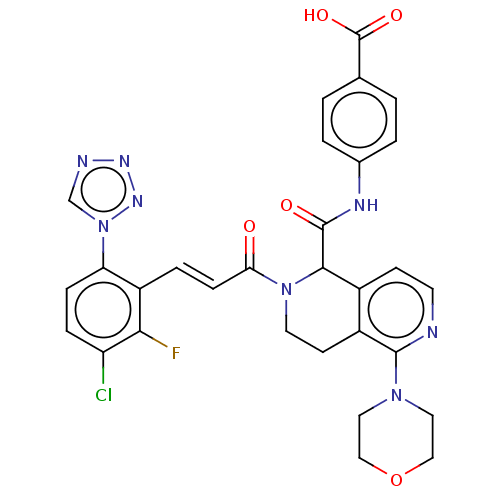 (US9108951, 28 | US9394276, 28 | US9725435, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161057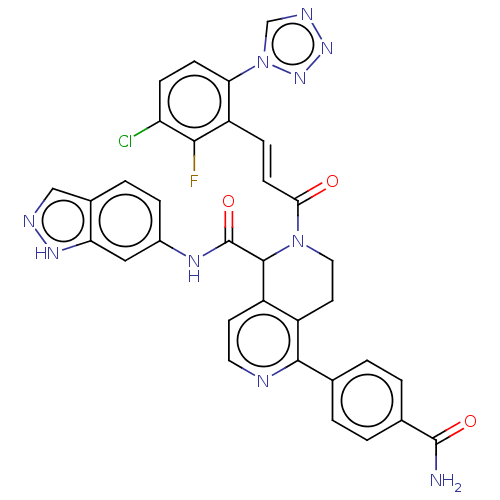 (US9108951, 23 | US9394276, 23 | US9725435, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM238219 (US9403774, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9403774 (2016) BindingDB Entry DOI: 10.7270/Q2CC0ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161065 (US9108951, 77 | US9394276, 77 | US9725435, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.02 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM222997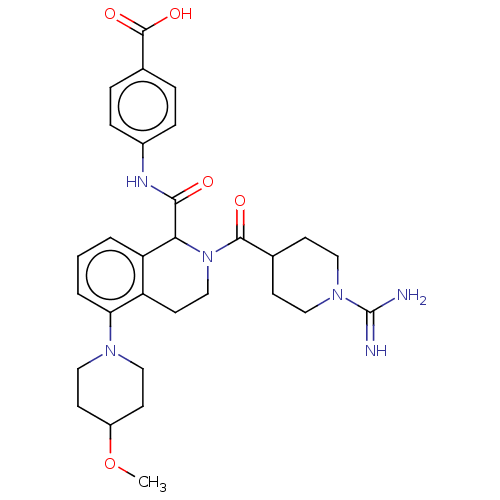 (US9315519, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.68 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9315519 (2016) BindingDB Entry DOI: 10.7270/Q2BC3XFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286020 ((E)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.24 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161061 (US9108951, 48 | US9394276, 48 | US9725435, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289805 (US10093683, Example 116A | US10093683, Example 116...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.51 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM131020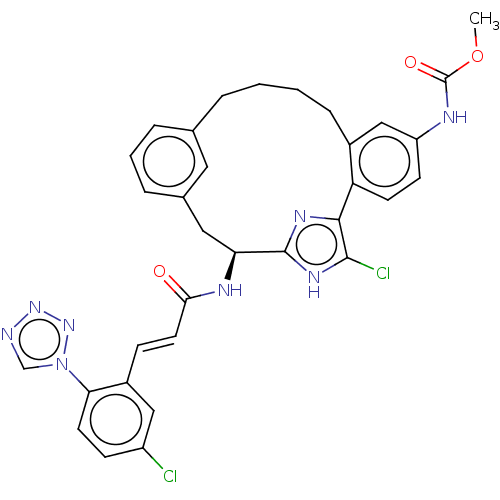 (US8828983, 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US8828983 (2014) BindingDB Entry DOI: 10.7270/Q2FB51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289849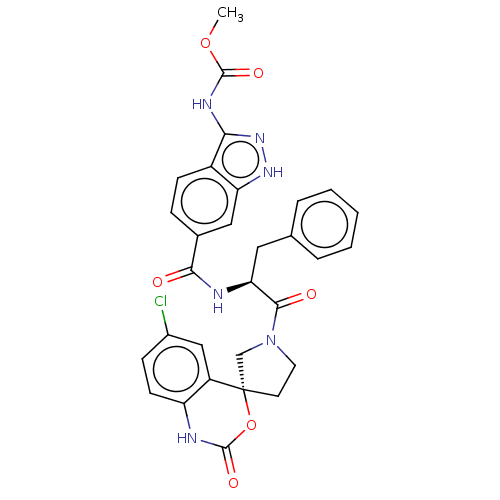 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289787 (US10093683, Example 14b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289811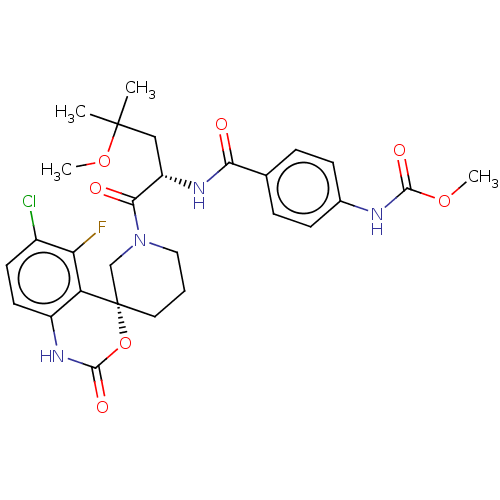 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.2 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161052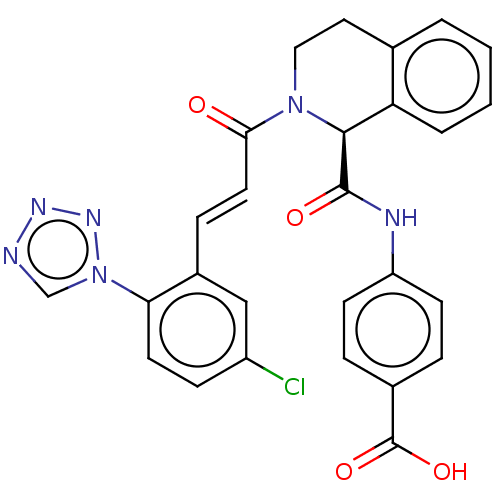 (US9108951, 3 | US9394276, 3 | US9725435, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289801 (Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286008 (US10081623, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289803 (N—((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.6 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM286019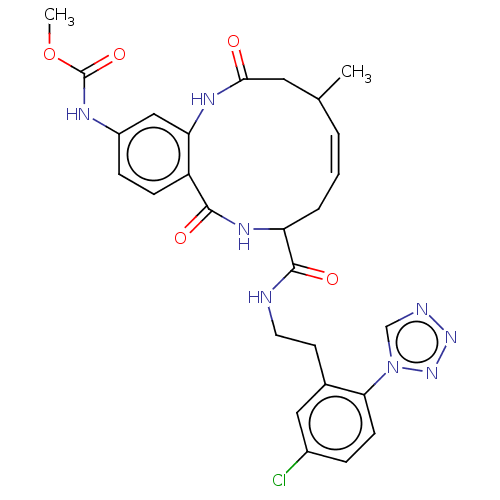 ((Z)-(+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285988 ((+/-)-Methyl N-[8-({2-[5-chloro-2-(1H-1,2,3,4-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm... | US Patent US10081623 (2018) BindingDB Entry DOI: 10.7270/Q24B33CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289848 (Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM161063 (US9108951, 65 | US9394276, 65 | US9725435, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9394276 (2016) BindingDB Entry DOI: 10.7270/Q2XG9Q1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289796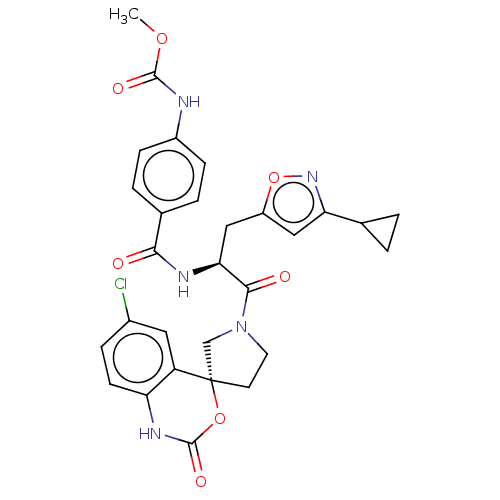 (Methyl(4-(((2S)-1-(6-chloro-2-oxo-1,2-dihydrospiro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.1 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289794 (US10093683, Example 21b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31.2 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 940 total ) | Next | Last >> |