

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM21358 (2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description The effect of compound on cytochrome P450 (CYP) enzyme catalytic activity was determined in human liver microsomes, using a cocktail of probe substra... | J Med Chem 50: 5535-8 (2007) Article DOI: 10.1021/jm070521y BindingDB Entry DOI: 10.7270/Q23R0R5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26467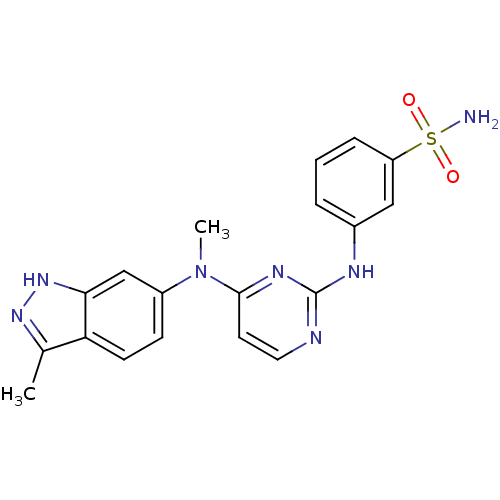 (3-({4-[methyl(3-methyl-1H-indazol-6-yl)amino]pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677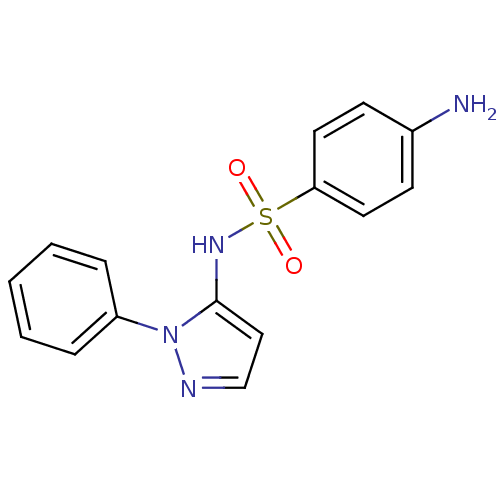 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM164620 (US9688624, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
NMMLSC US Patent | Assay Description Specific aspects of the incubation conditions for each assay (e.g., protein concentration, incubation time, etc.) are defined in Walsky & Obach, 2004... | US Patent US9688624 (2017) BindingDB Entry DOI: 10.7270/Q2416V6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM106203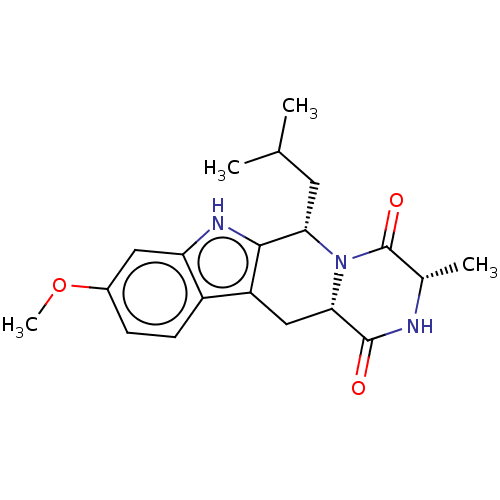 (US9695174, I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50305083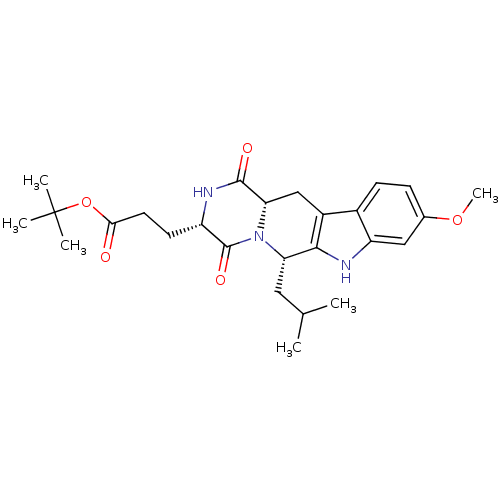 (3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Millennium Pharmaceuticals, Inc. US Patent | Assay Description Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective sub... | US Patent US9695174 (2017) BindingDB Entry DOI: 10.7270/Q2DN436B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM7841 (BAY 59-7939 Analog 18 | US8822458, 45 | US9359341,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
WANBURY LTD. US Patent | Assay Description Incubation of phenacetin, diclofenac, dextromethorphan, mephenotoin, albendazole and testosterone with human liver microsomes in the presence of in e... | US Patent US9359341 (2016) BindingDB Entry DOI: 10.7270/Q29Z93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM235143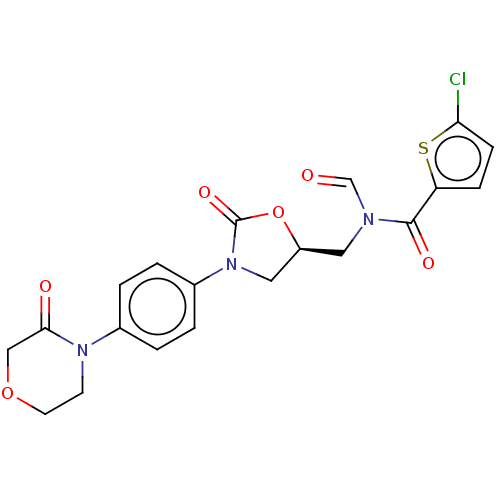 (US9359341, Formula B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
WANBURY LTD. US Patent | Assay Description Incubation of phenacetin, diclofenac, dextromethorphan, mephenotoin, albendazole and testosterone with human liver microsomes in the presence of in e... | US Patent US9359341 (2016) BindingDB Entry DOI: 10.7270/Q29Z93S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM155255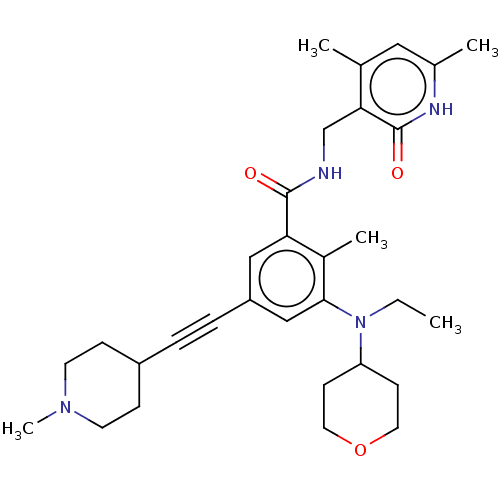 (US10098888, Compound 105 | US11642348, Compound 10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.57E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26468 (3-({4-[(1,3-dimethyl-1H-indazol-6-yl)(methyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26469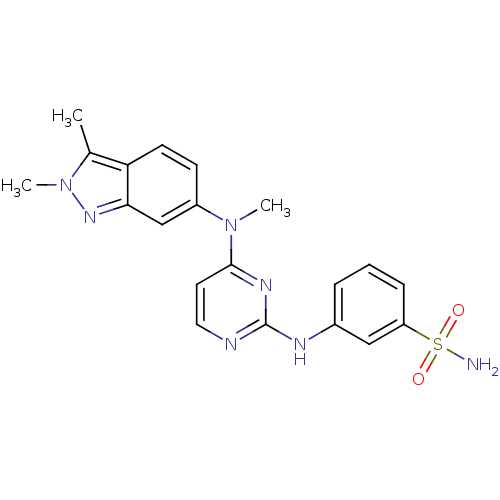 (3-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26470 (3-({4-[(2-benzyl-3-methyl-2H-indazol-6-yl)(methyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26471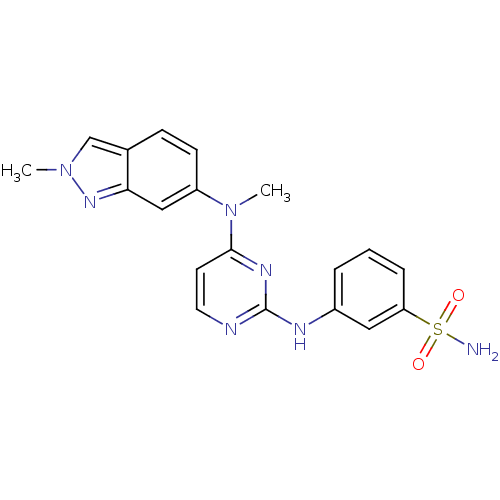 (3-({4-[methyl(2-methyl-2H-indazol-6-yl)amino]pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26472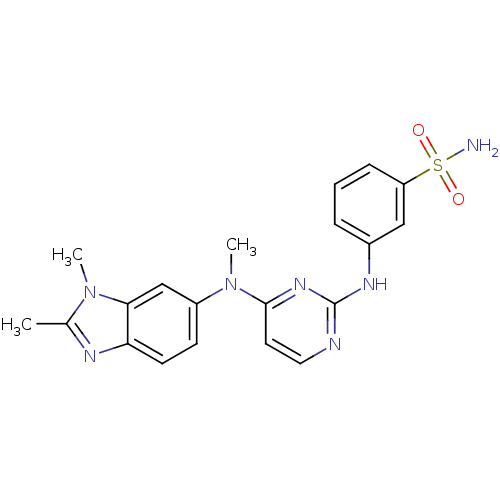 (3-({4-[(1,2-dimethyl-1H-1,3-benzodiazol-6-yl)(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM26473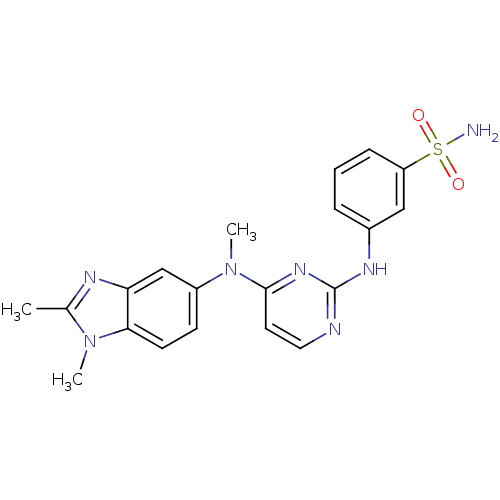 (3-({4-[(1,2-dimethyl-1H-1,3-benzodiazol-5-yl)(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GSK | Assay Description A range of concentrations of each compound in a 96-well plate were preincubated in buffer containing recombinant human CYP450 microsomal protein and ... | J Med Chem 51: 4632-40 (2008) Article DOI: 10.1021/jm800566m BindingDB Entry DOI: 10.7270/Q26971XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM155253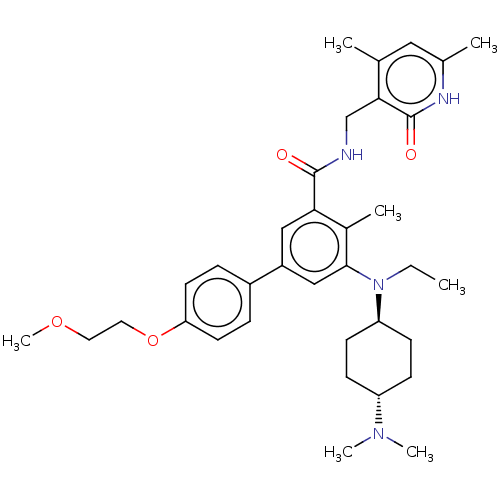 (US10098888, Compound 1 | US9006242, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM155254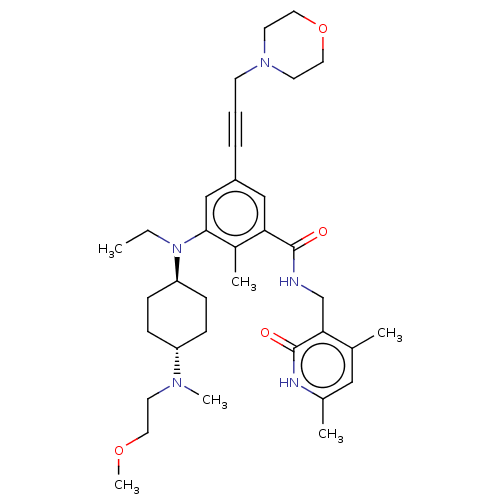 (US10098888, Compound 2 | US9006242, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Epizyme, Inc. US Patent | Assay Description The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... | US Patent US9006242 (2015) BindingDB Entry DOI: 10.7270/Q2222SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50120439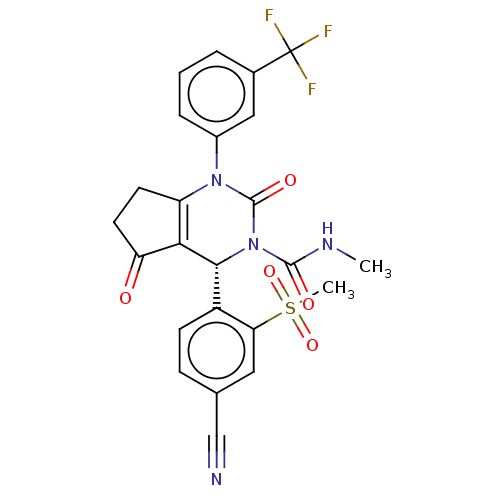 (CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM211836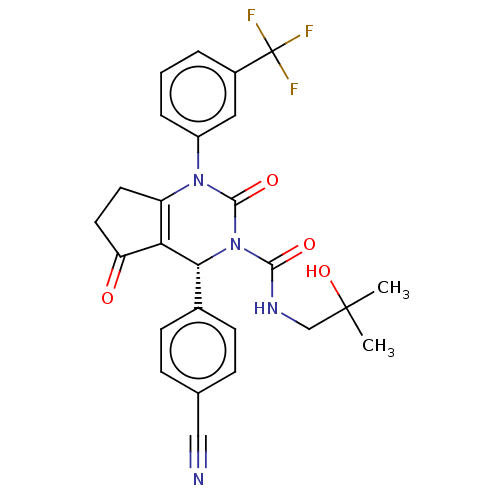 (US9290459, 41A | US9670166, 41A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM211750 (US9290459, 7.2A | US9670166, 7.2A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM211754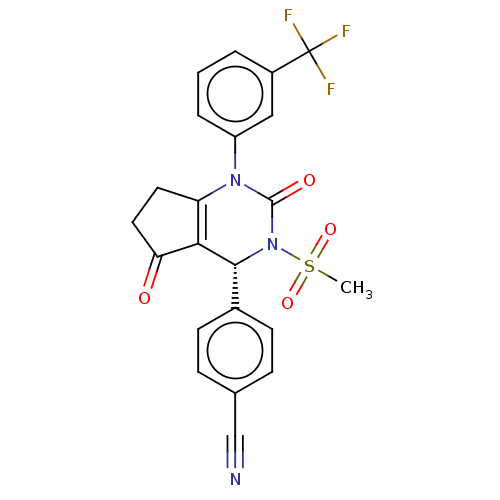 (US9290459, 9A | US9670166, 9A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM211757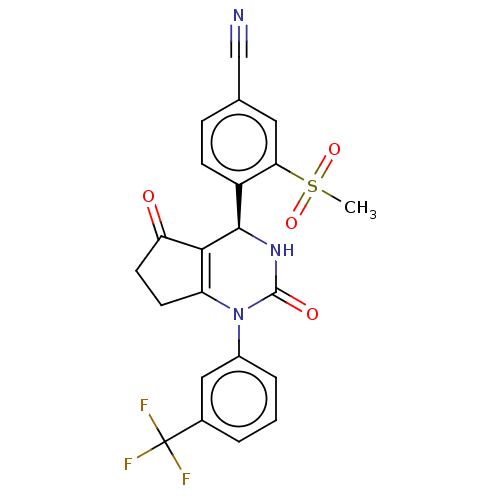 (US9290459, 10A | US9290459, 47.21 | US9670166, 10A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50120423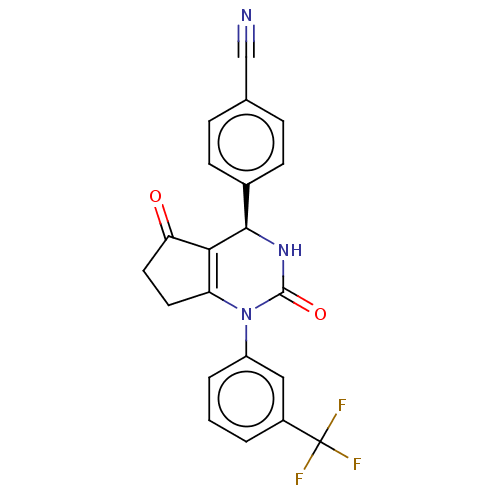 (CHEMBL3617971 | US9290459, 1A | US9670166, 1A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM211887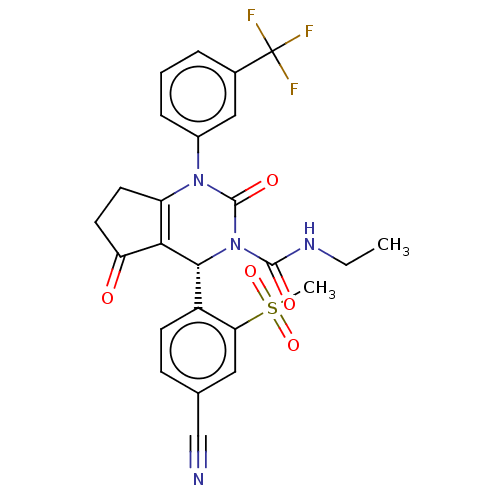 (US9290459, 47.2 | US9670166, 47.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM191369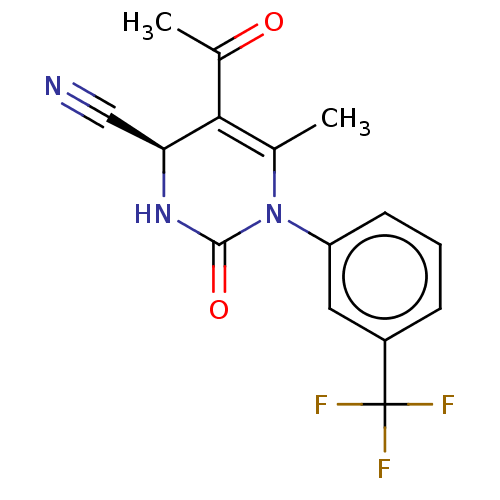 (WO2005082863, Example 8A | example 8A disclosed in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9670166 (2017) BindingDB Entry DOI: 10.7270/Q25Q4T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||