

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Legumain (Homo sapiens (Human)) | BDBM228617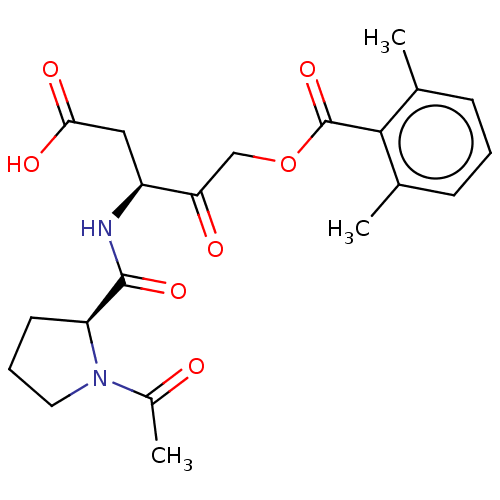 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM228617 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 704 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM36333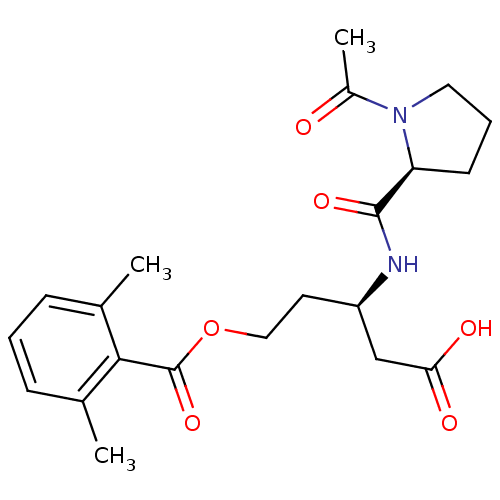 (LI-0 | Legumain Inhibitor -0) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 704 | n/a | n/a | n/a | n/a | 5.8 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM36332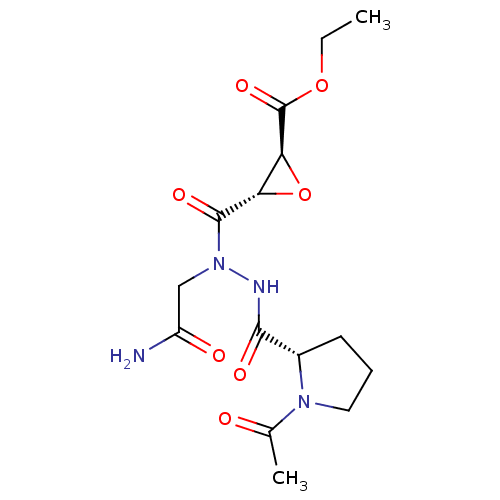 (LI-1 | Legumain Inhibitor -1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 5.8 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM228617 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 145 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22121 (aza-peptide Michael acceptor, 5e | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22122 (aza-peptide Michael acceptor, 5f | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22123 (aza-peptide Michael acceptor, 5g | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22124 (aza-peptide Michael acceptor, 5h | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22125 (aza-peptide Michael acceptor, 5i | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22126 (aza-peptide Michael acceptor, 5j | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22127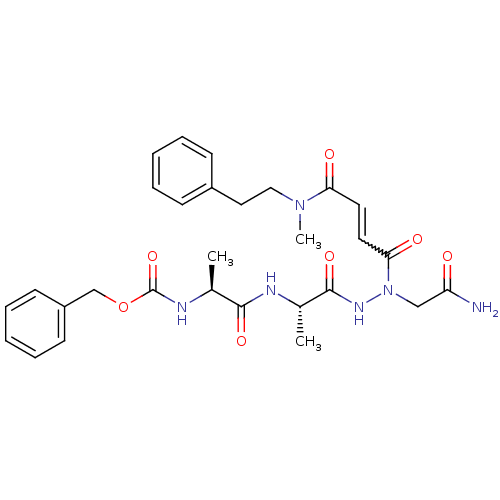 (aza-peptide Michael acceptor, 5k | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22128 (aza-peptide Michael acceptor, 5l | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22129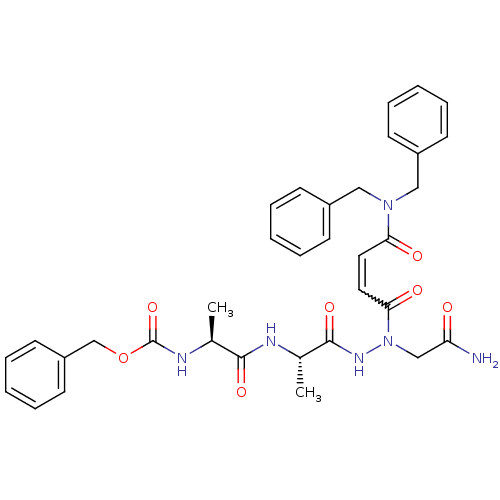 (aza-peptide Michael acceptor, 5m | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22130 (aza-peptide Michael acceptor, 5n | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22131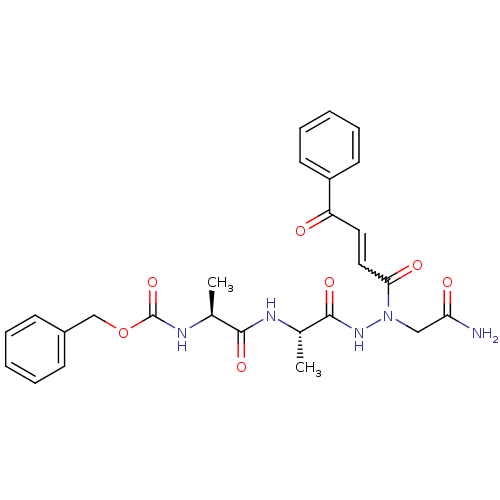 (aza-peptide Michael acceptor, 7c | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22132 (aza-peptide Michael acceptor, 7d | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22133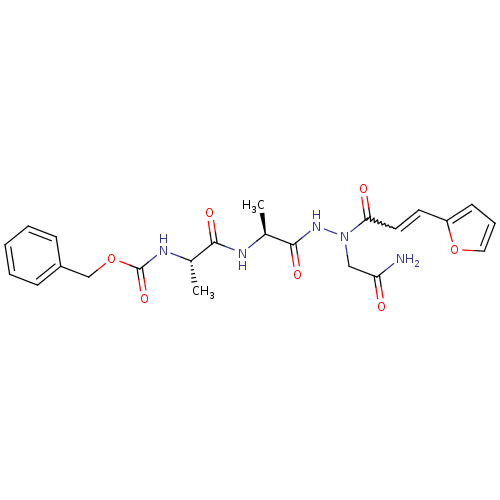 (aza-peptide Michael acceptor, 7e | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22134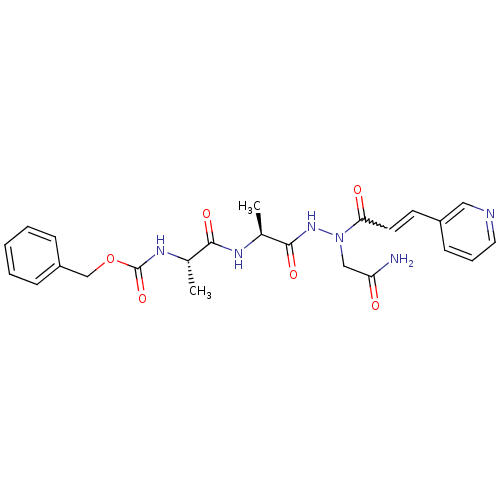 (aza-peptide Michael acceptor, 7f | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22120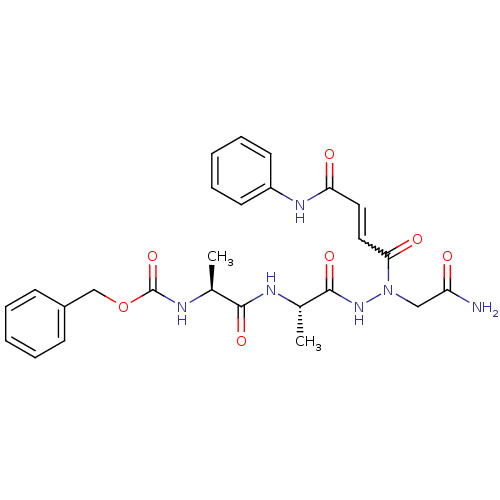 (aza-peptide Michael acceptor, 5d | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22119 (aza-peptide Michael acceptor, 5c | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22118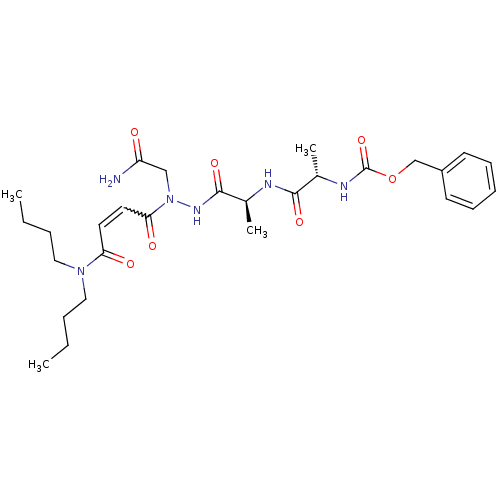 (aza-peptide Michael acceptor, 5b | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22117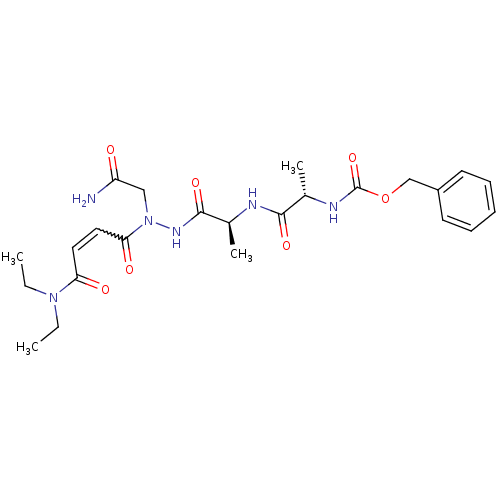 (aza-peptide Michael acceptor, 5a | benzyl N-[(1S)-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22114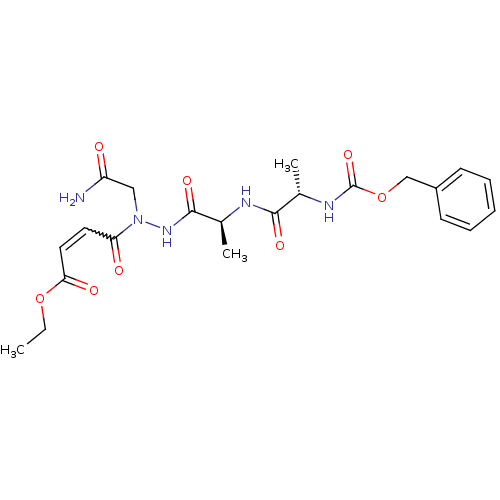 (aza-peptide Michael acceptor, 7a | ethyl (2E)-4-[(...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Schistosoma mansoni) | BDBM22116 (aza-peptide Michael acceptor, 7b | benzyl (2E)-3-{...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Georgia Institute of Technology | Assay Description AE activity in the induction medium was measured with the substrate Cbz-Ala-Ala-Asn-AMC. In a black 96-well microtiter plate, aza-peptidyl inhibitors... | J Med Chem 51: 2816-32 (2008) Article DOI: 10.1021/jm701311r BindingDB Entry DOI: 10.7270/Q2KK9937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||