

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50002966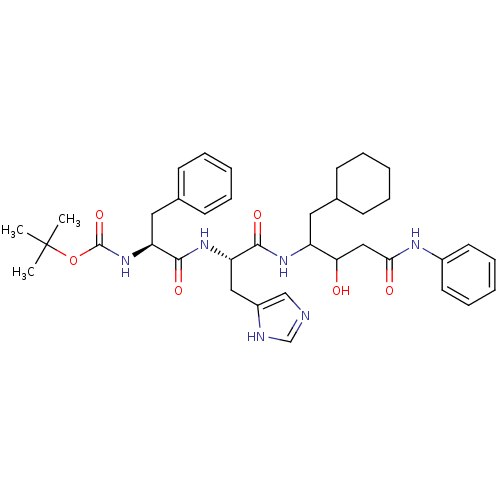 (CHEMBL115430 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002990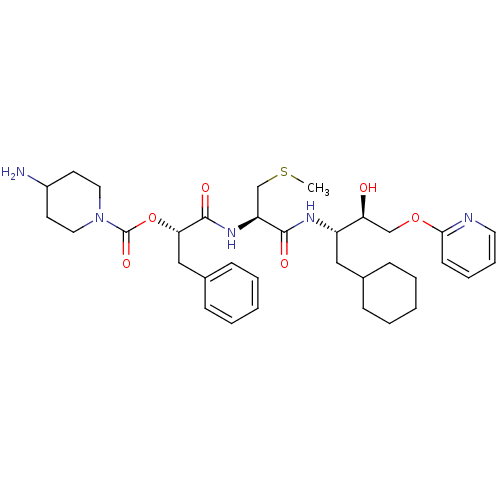 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002970 (4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002972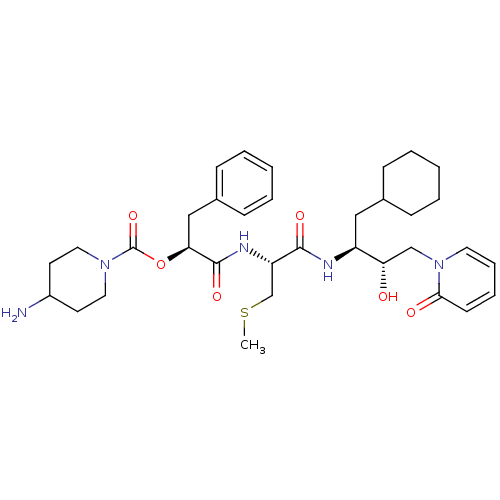 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002974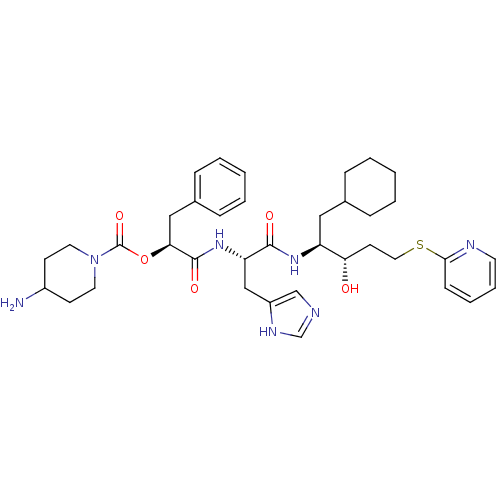 (4-Amino-piperidine-1-carboxylic acid 1-[1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002971 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002977 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002978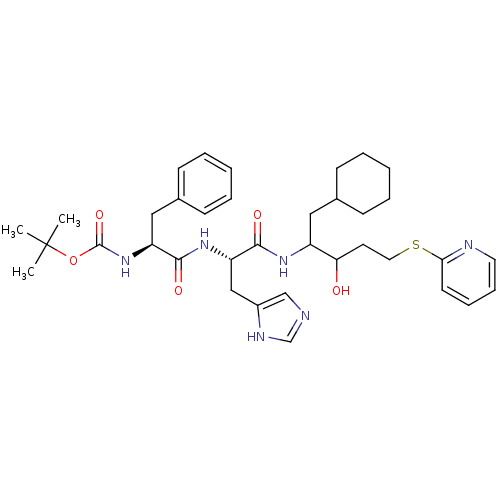 (CHEMBL119893 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002982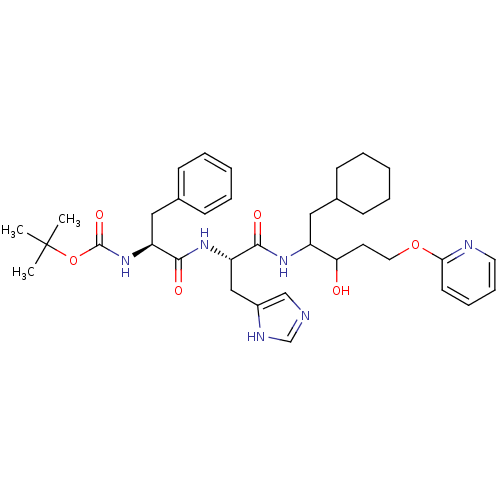 (CHEMBL333707 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002975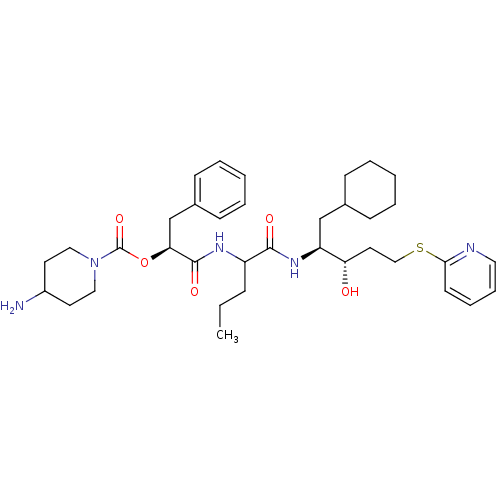 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002969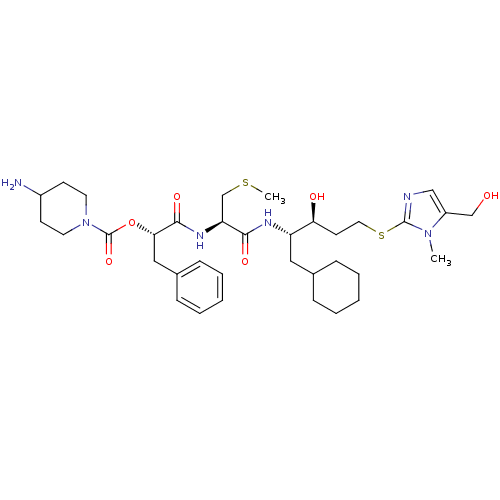 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002984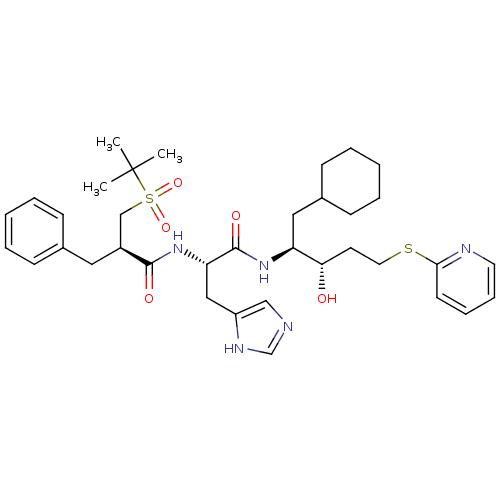 (2-Benzyl-N-[1-[1-cyclohexylmethyl-2-hydroxy-4-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002986 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002980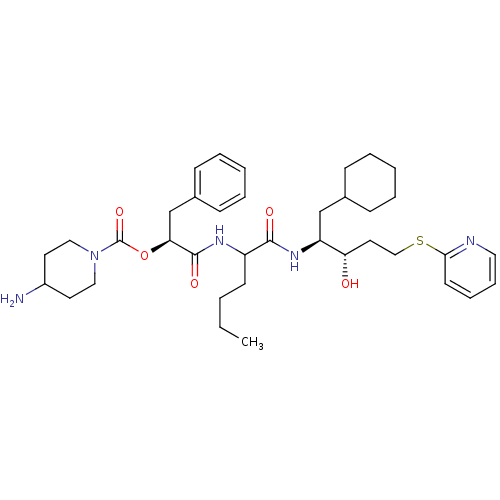 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002988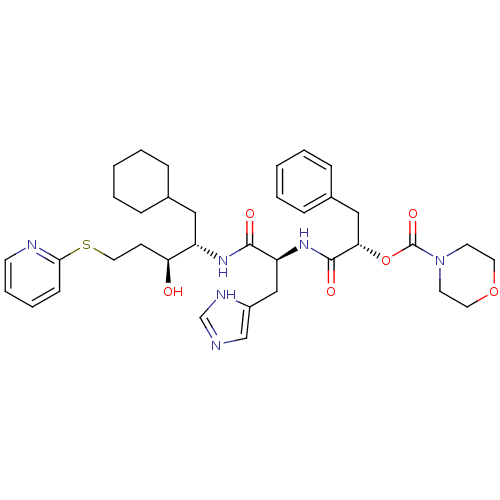 (CHEMBL118172 | Morpholine-4-carboxylic acid 1-[1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002979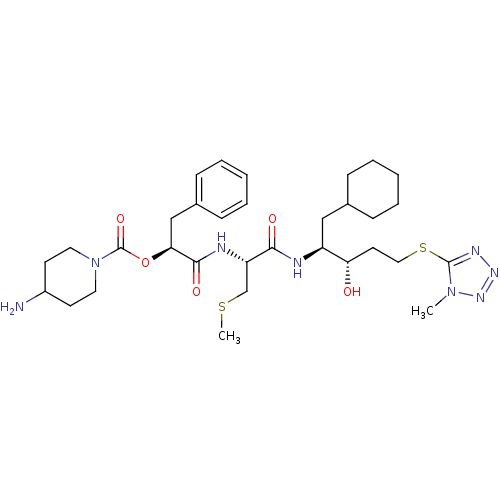 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002987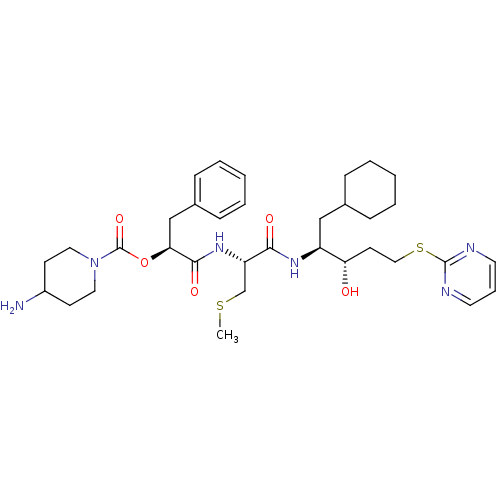 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002981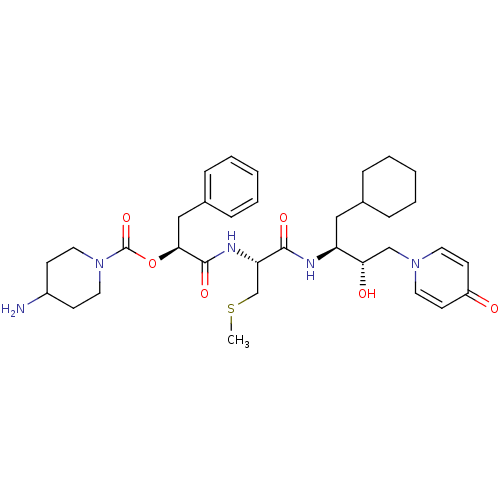 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002991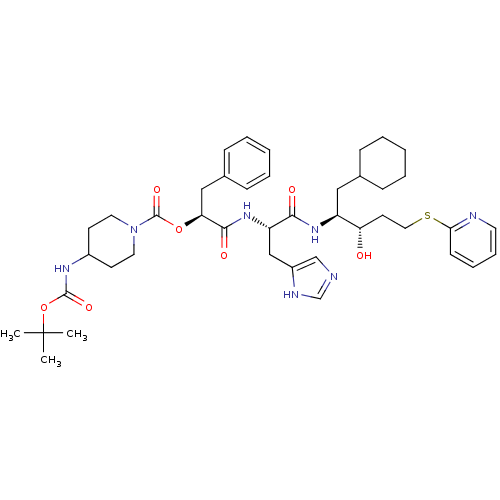 (4-tert-Butoxycarbonylamino-piperidine-1-carboxylic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002993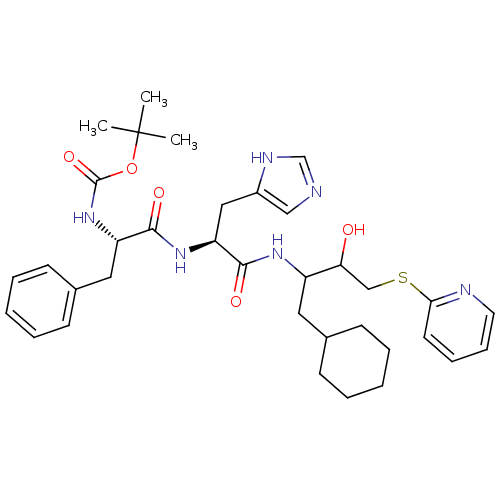 (CHEMBL334319 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002992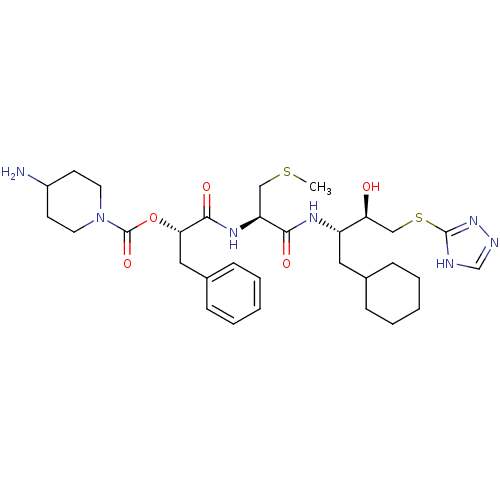 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002976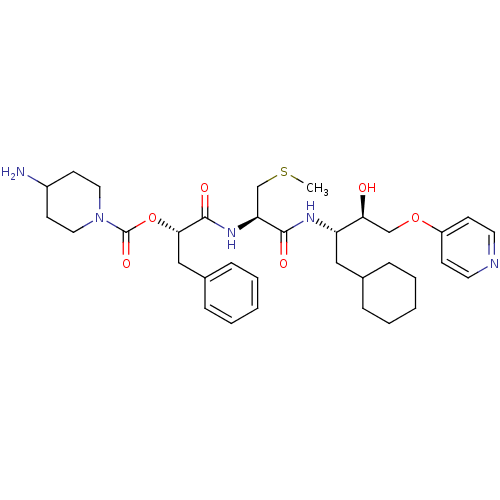 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002989 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002973 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002967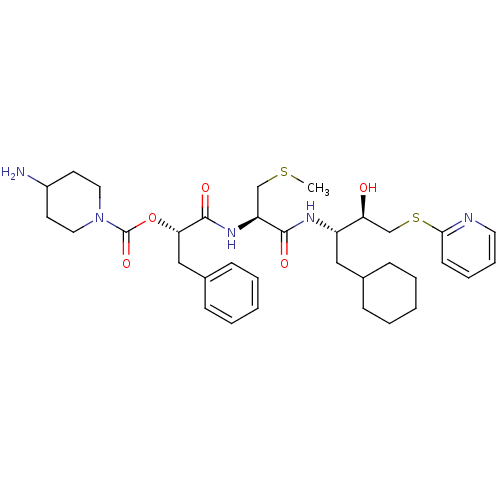 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002985 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002994 (2-Benzyl-N-[1-[1-cyclohexylmethyl-2-hydroxy-4-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002995 (CHEMBL327068 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002983 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002968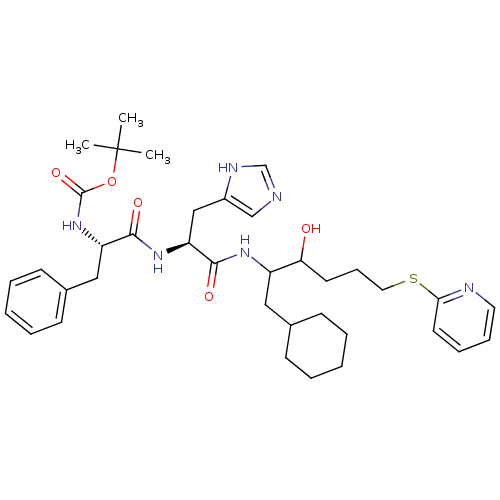 (CHEMBL117799 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50405191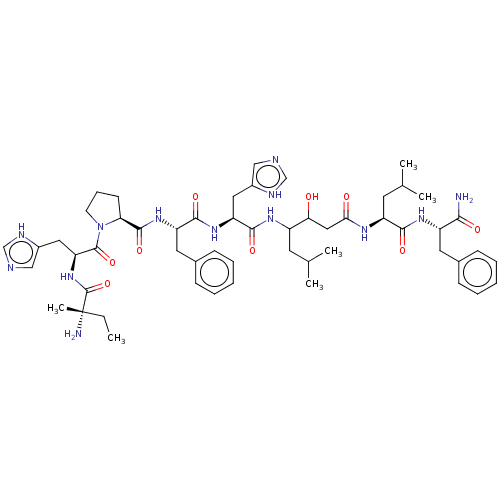 (CHEMBL269752) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of rat plasma renin at pH of 6.0. | J Med Chem 28: 1779-90 (1986) BindingDB Entry DOI: 10.7270/Q2FJ2HC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280235 ((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at PH 6.0 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280224 ((1S,2R,3S)-2-Methanesulfonyl-3-phenyl-cyclopropane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at PH 6.0 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280236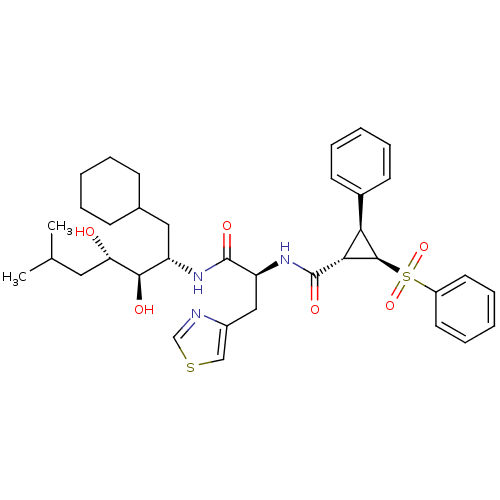 ((1S,2R,3S)-2-Benzenesulfonyl-3-phenyl-cyclopropane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at PH 6.0 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280228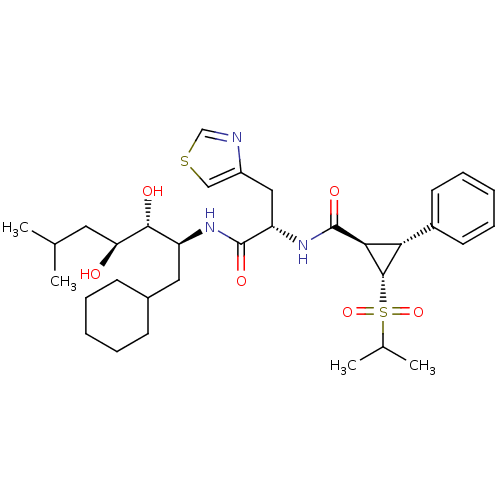 ((1R,2R,3S)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at PH 6.0 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280221 ((1S,2S,3R)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of human plasma renin at PH 7.4 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280238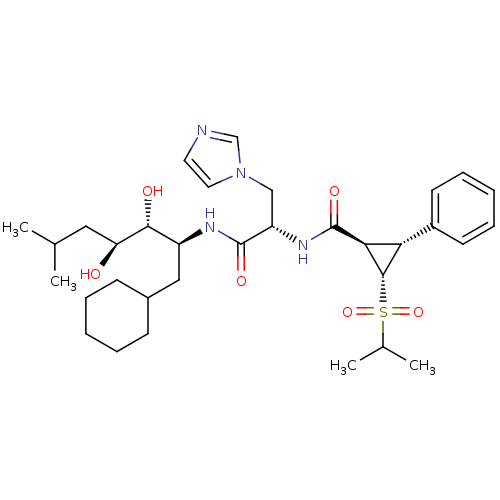 ((1R,2R,3S)-2-Phenyl-3-(propane-2-sulfonyl)-cyclopr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of human plasma renin at PH 7.4 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280227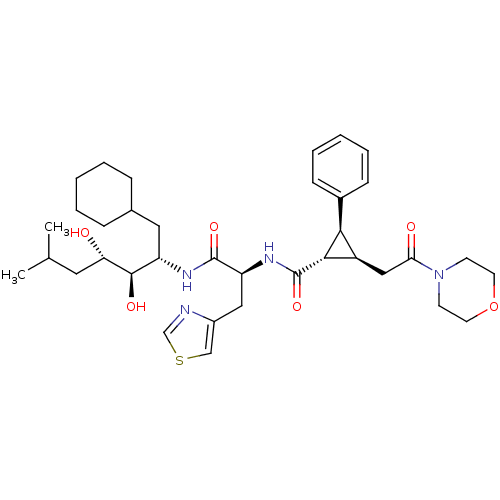 ((1S,2R,3S)-2-(2-Morpholin-4-yl-2-oxo-ethyl)-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at PH 6.0 | Bioorg Med Chem Lett 2: 1405-1410 (1992) Article DOI: 10.1016/S0960-894X(00)80522-3 BindingDB Entry DOI: 10.7270/Q2VD6ZBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006119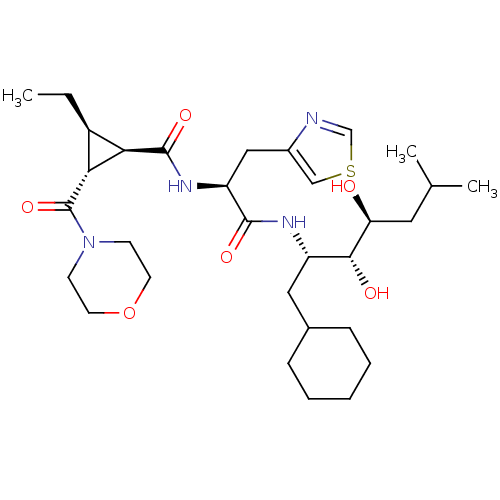 (2-Ethyl-3-(morpholine-4-carbonyl)-cyclopropanecarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at pH 7.4 | J Med Chem 35: 1710-21 (1992) BindingDB Entry DOI: 10.7270/Q2GT5M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006138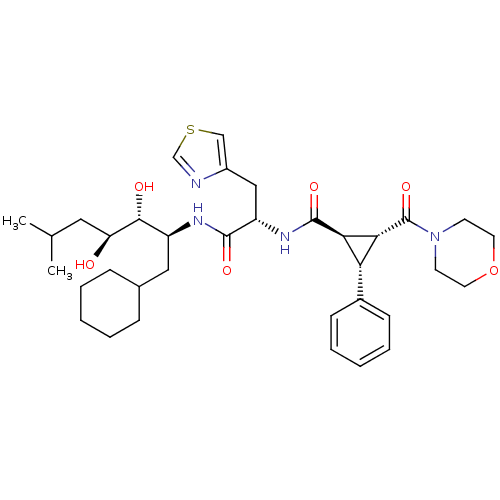 (2-(Morpholine-4-carbonyl)-3-phenyl-cyclopropanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at pH 7.4 | J Med Chem 35: 1710-21 (1992) BindingDB Entry DOI: 10.7270/Q2GT5M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006124 (2-Ethyl-3-(morpholine-4-carbonyl)-cyclopropanecarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas Curated by ChEMBL | Assay Description In vitro potency against human plasma renin at pH 7.4 | J Med Chem 35: 1710-21 (1992) BindingDB Entry DOI: 10.7270/Q2GT5M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006134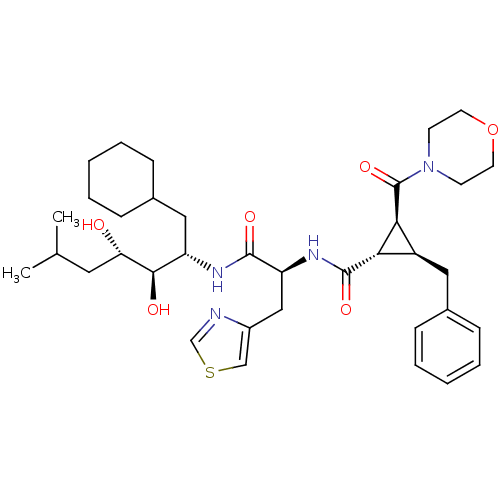 (2-Benzyl-3-(morpholine-4-carbonyl)-cyclopropanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Texas Curated by ChEMBL | Assay Description In vitro inhibition of purified human renin at a pH of 6.0. | J Med Chem 35: 1710-21 (1992) BindingDB Entry DOI: 10.7270/Q2GT5M4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006160 (2-(1-Benzyl-2-morpholin-4-yl-2-oxo-ethylamino)-N-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006146 (2-[1-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 6.0. | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006158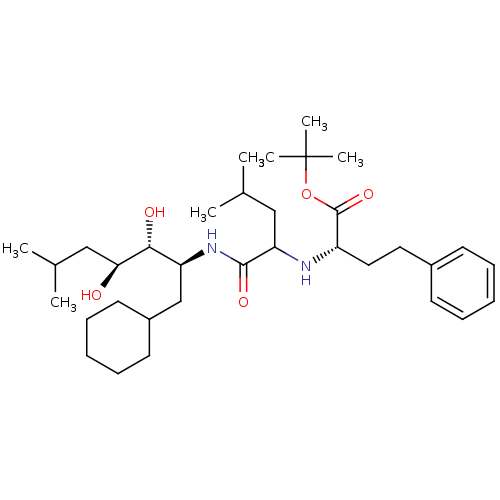 (2-[1-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006142 (2-(1-Benzyl-2-morpholin-4-yl-2-oxo-ethylamino)-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 6.0 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006161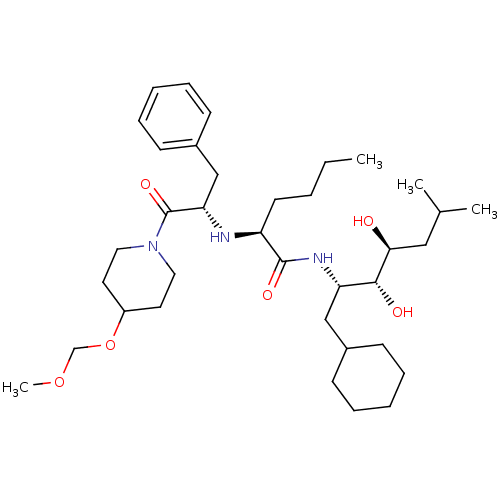 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 6.0. | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006174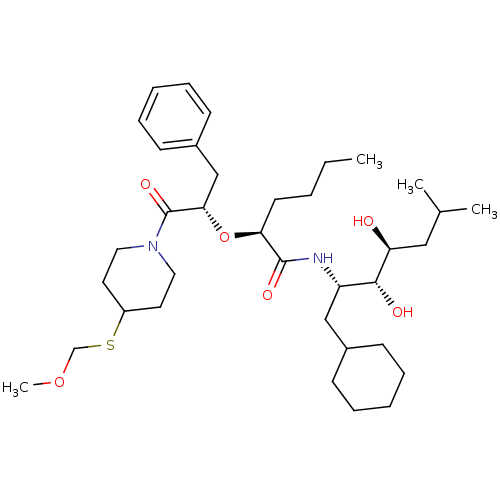 (2-[1-Benzyl-2-(4-methoxymethylsulfanyl-piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006152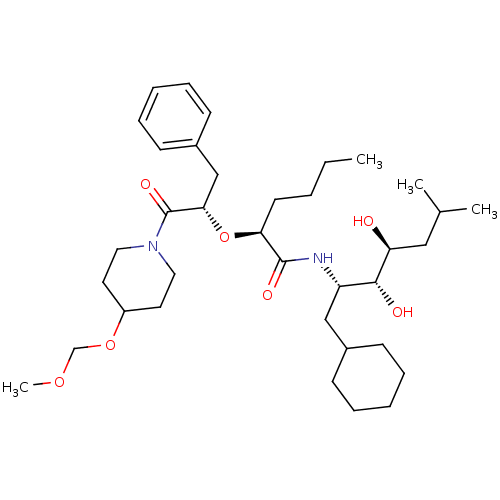 (2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against plasma renin at a pH of 7.4 | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006163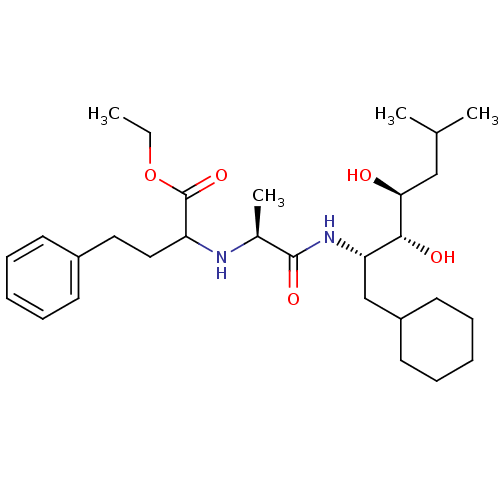 (2-[1-(1-Cyclohexylmethyl-2,3-dihydroxy-5-methyl-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro potency against purified renin at a pH of 6.0. | J Med Chem 35: 1722-34 (1992) BindingDB Entry DOI: 10.7270/Q2C24VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 902 total ) | Next | Last >> |