

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263548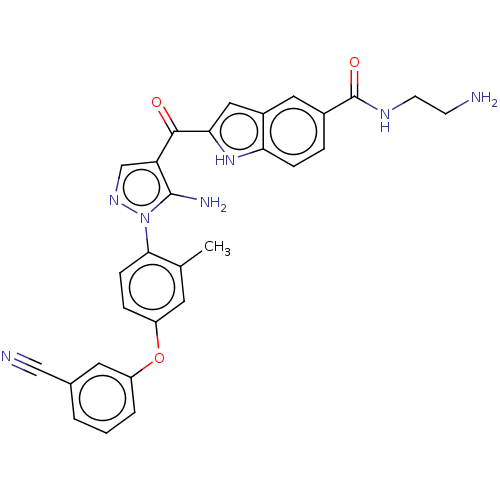 (2-{5-amino-1-[4-(3-cyano- phenoxy)-2-methyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263549 (3-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263550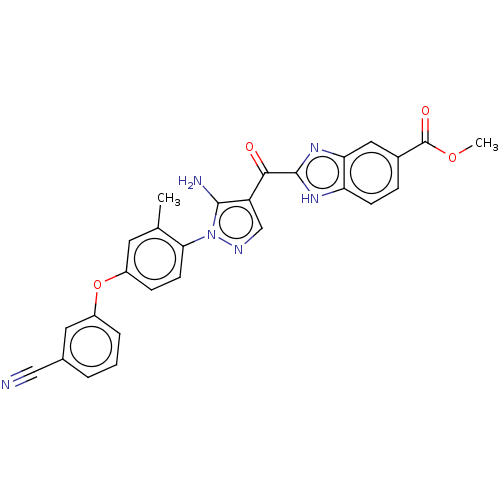 (2-{5-amino-1-[4-(3-cyano- phenoxy)-2-methyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263551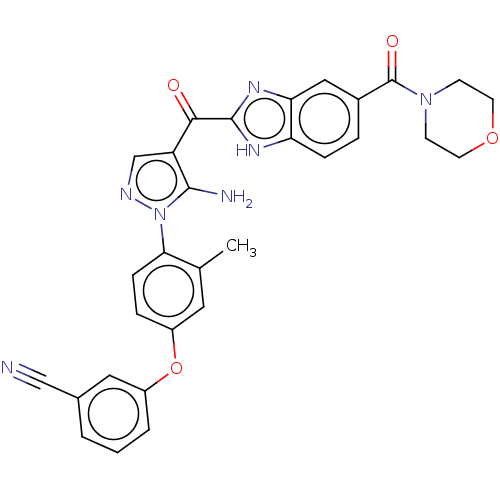 (3-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263552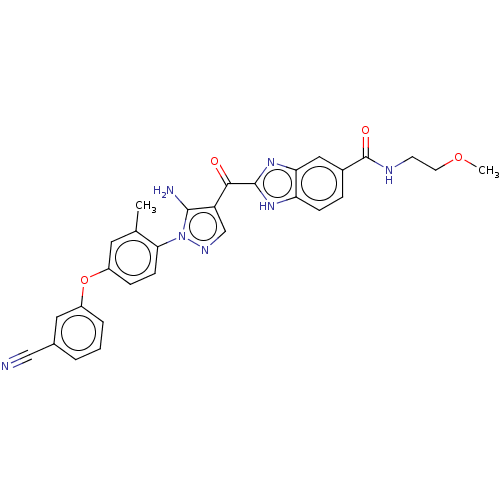 (2-{5-amino-1-[4-(3-cyano- phenoxy)-2-methyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263553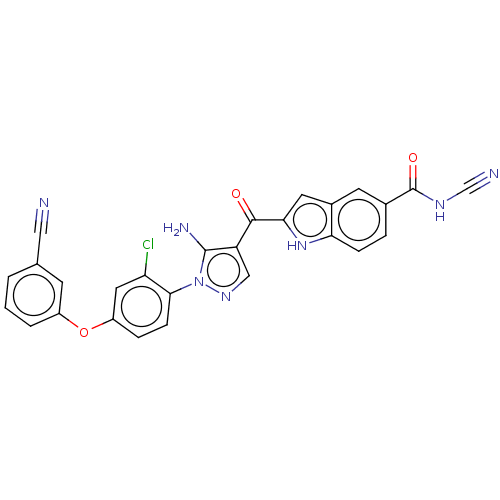 (2-{5-amino-1-[2-chloro-4-(3- cyano-phenoxy)-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263554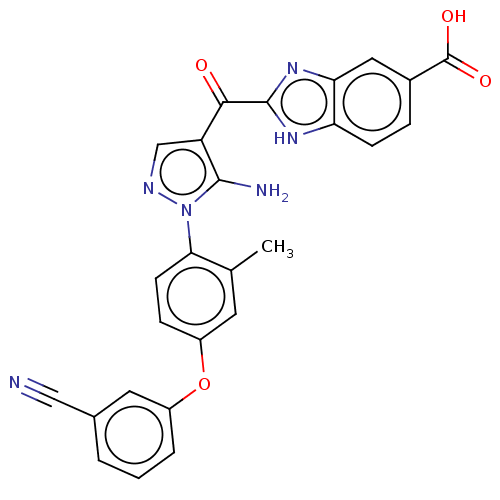 (2-{5-amino-1-[4-(3-cyano- phenoxy)-2-methyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263555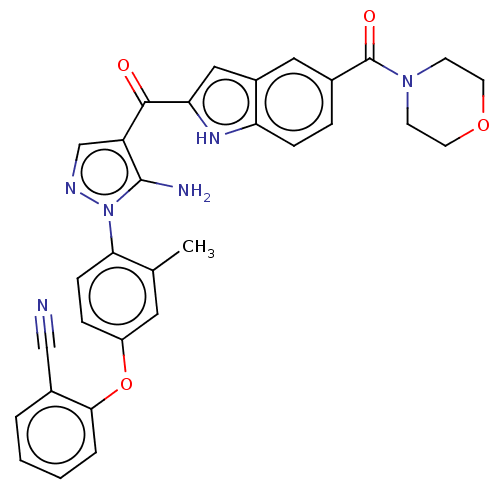 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263556 (3-(4-{5-amino-4-[5-(4-methyl- piperazine-1-carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263557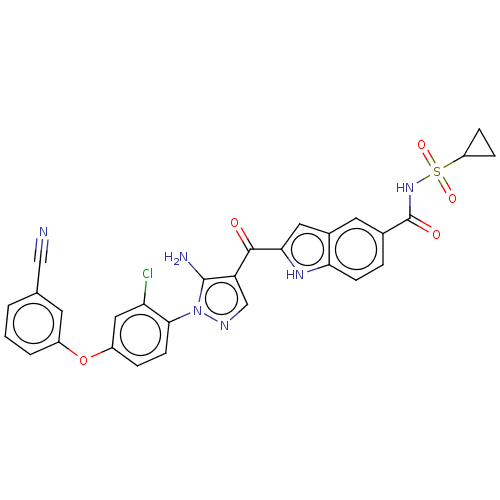 (US9556150, i-45 | cyclopropanesulfonic acid (2-{5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263558 (2-(4-{5-amino-4-[5-(4-methyl- piperazine-1-carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263559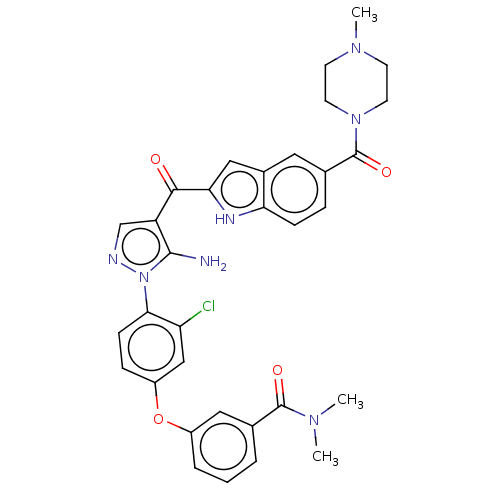 (3-(4-{5-amino-4-[5-(4-methyl- piperazine-1-carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263560 (2-{5-amino-1-[2-chloro-4-(3- cyano-2-fluoro-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263561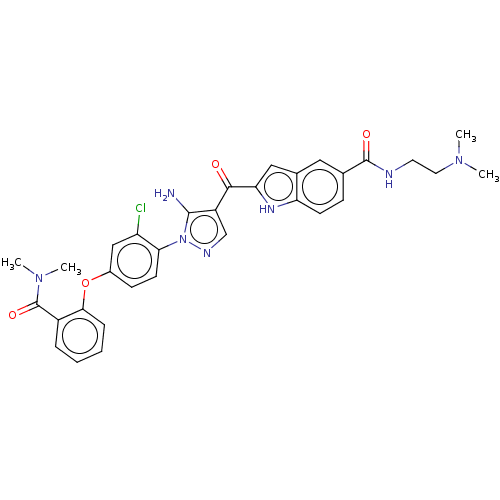 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263562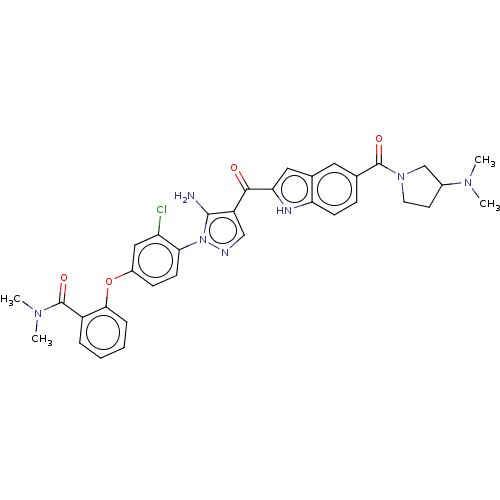 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263563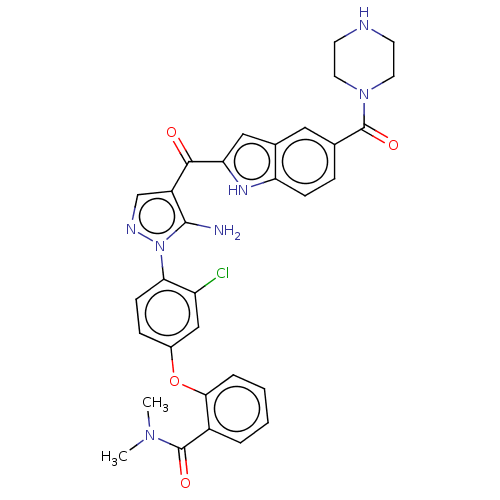 (2-(4-{5-amino-4-[5-(piperazine- 1-carbonyl)-1h-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263564 (US9556150, i-52 | [1-(2-{5-amino-1-[2-chloro-4- (2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263565 (2-(4-{5-amino-4-[5-(3- dimethylamino-pyrrolidine-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263566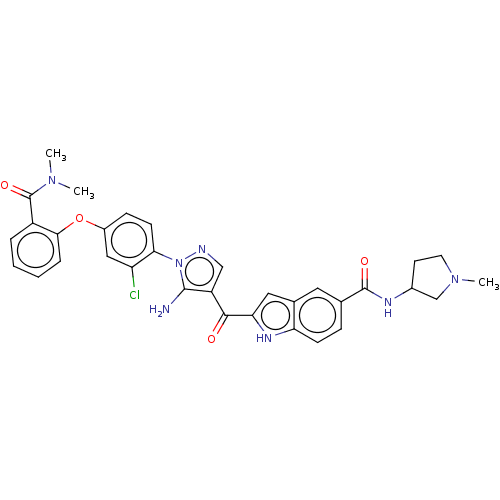 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263567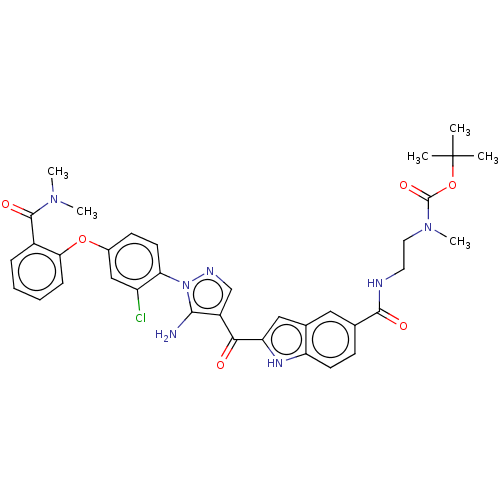 (US9556150, i-55 | {2-[(2-{5-amino-1-[2-chloro-4- (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263568 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263569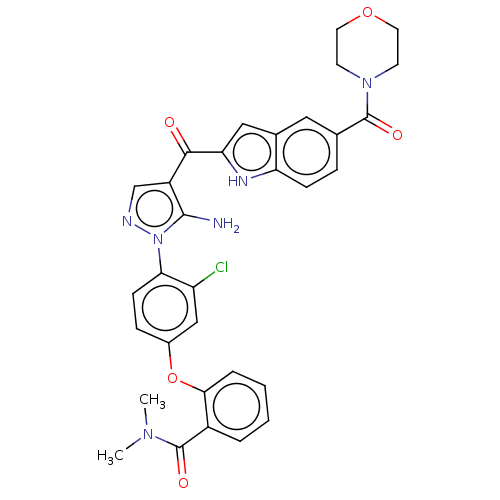 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263570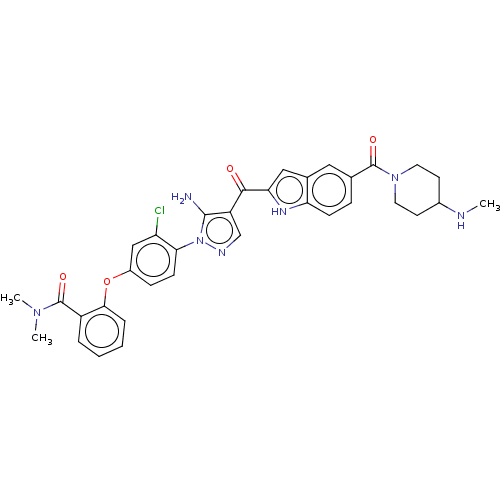 (2-(4-{5-amino-4-[5-(4- methylamino-piperidine-1- c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263571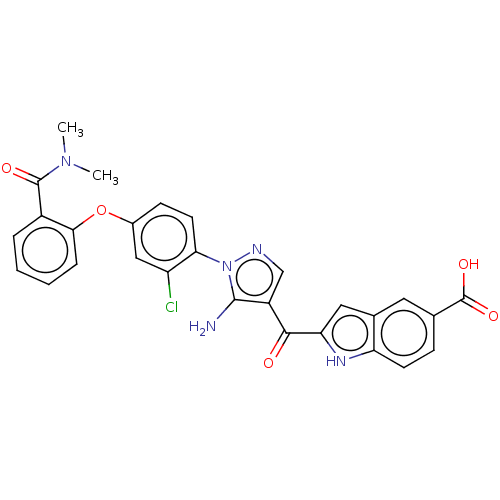 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235155 (US9359345, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 254 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235156 (US9359345, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.74E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235157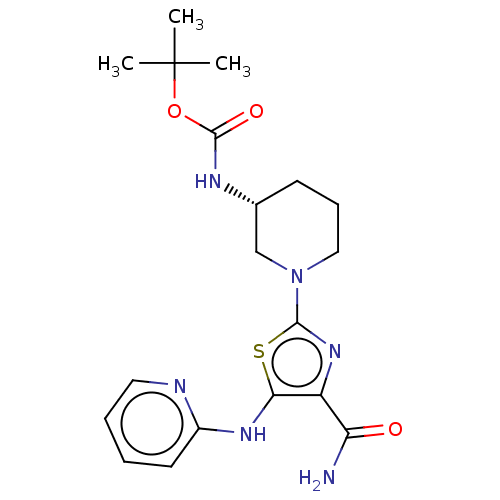 (US9359345, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235158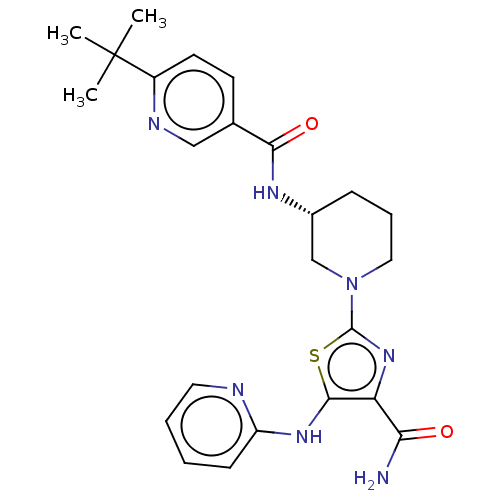 (US9359345, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235159 (US9359345, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235160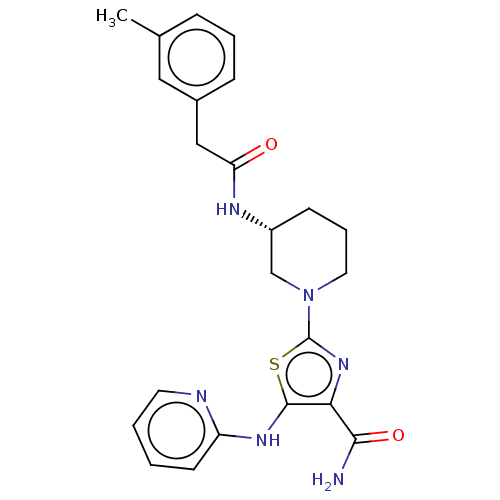 (US9359345, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.51E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235161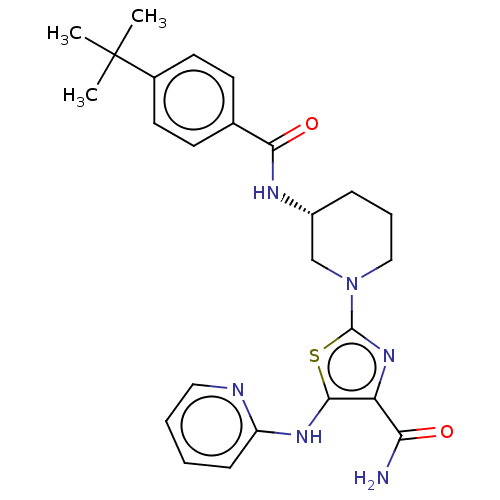 (US9359345, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235162 (US9359345, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.07E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235163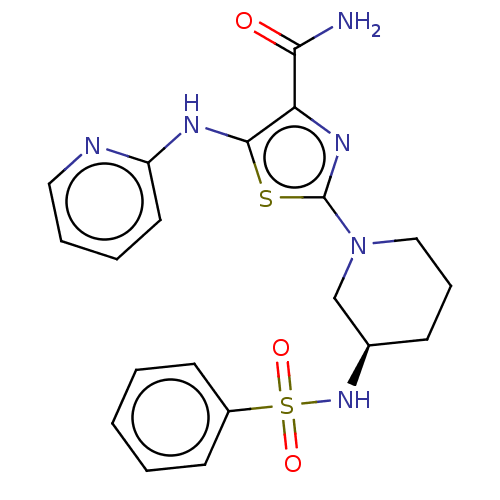 (US9359345, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235164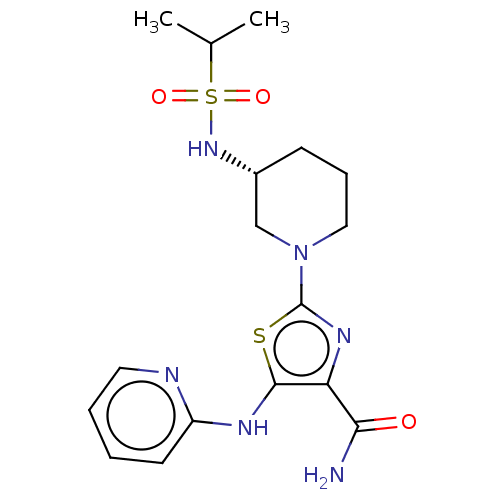 (US9359345, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235165 (US9359345, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM235166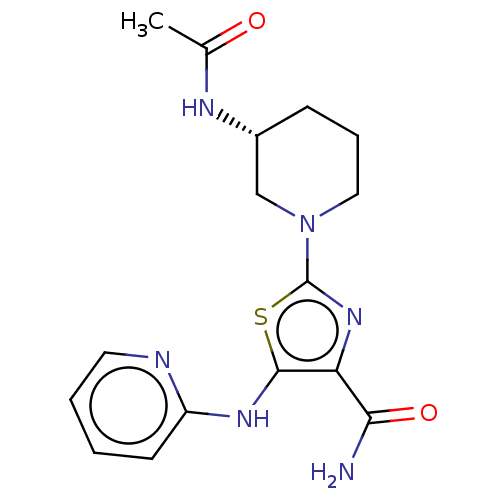 (US9359345, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.03E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9359345 (2016) BindingDB Entry DOI: 10.7270/Q2668C21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250515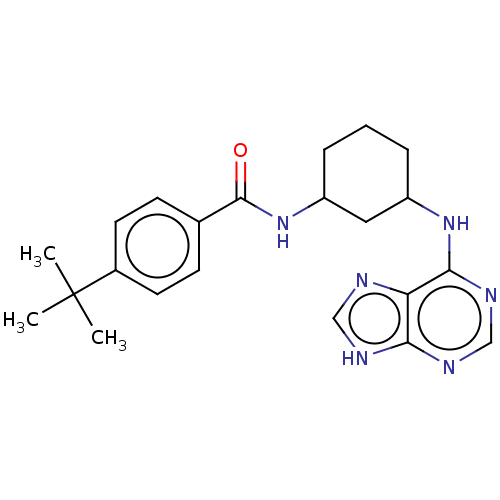 (US9469644, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 9.57E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250516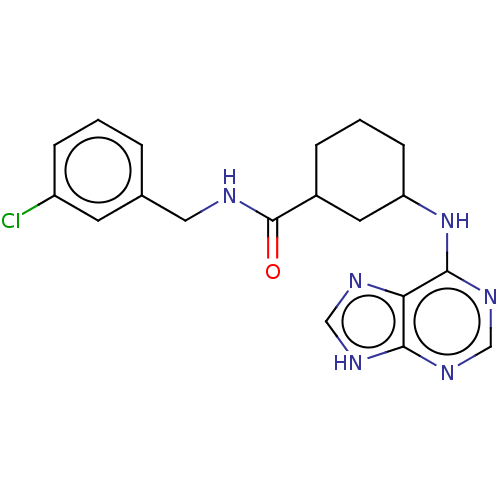 (US9469644, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250517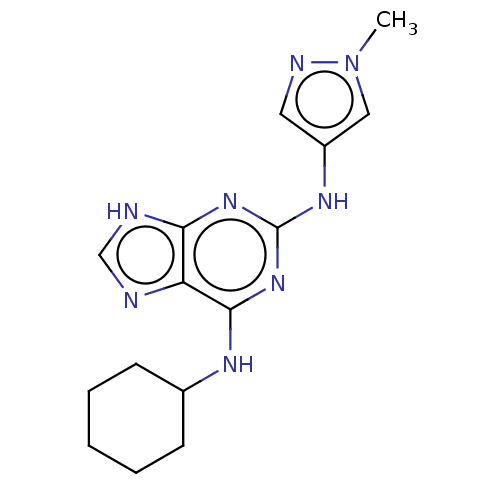 (US9469644, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250518 (US9469644, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.06E+4 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250519 (US9469644, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56.9 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250520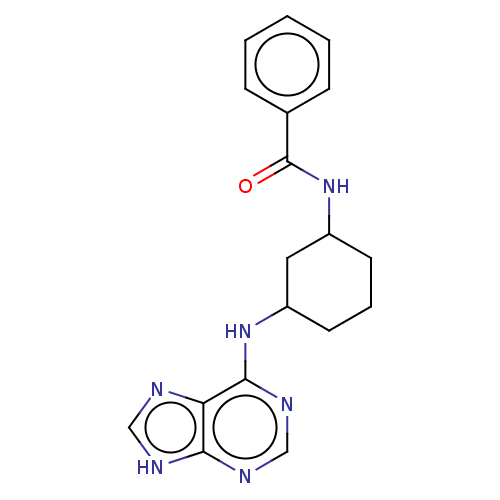 (US9469644, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 45.3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM250521 (US9469644, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 109 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (FOrster/Flouresence Res... | US Patent US9469644 (2016) BindingDB Entry DOI: 10.7270/Q24Q7SXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249203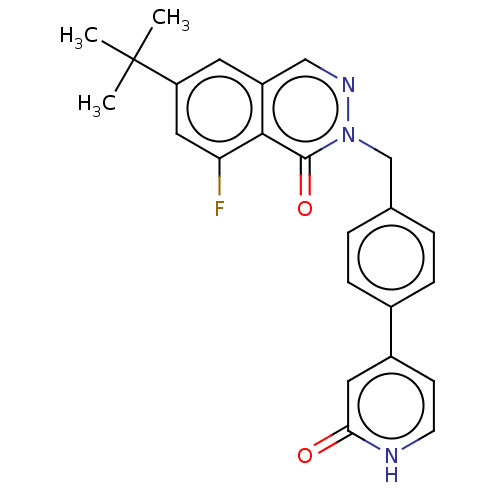 (US9458105, I-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249204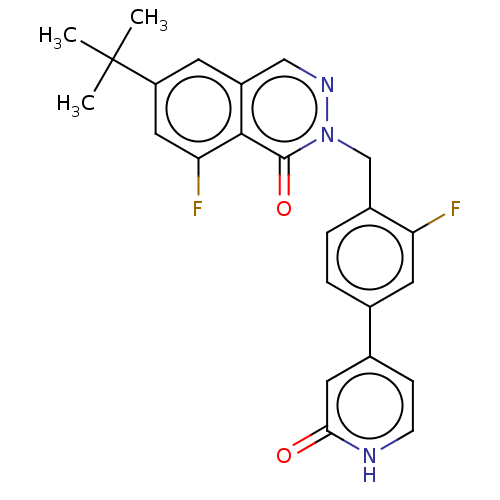 (US9458105, I-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 983 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249205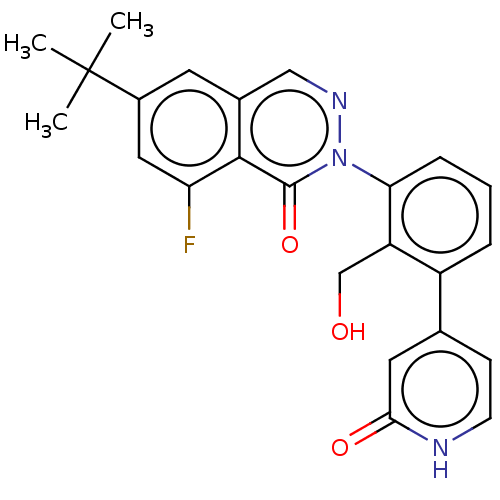 (US9458105, I-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 366 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249206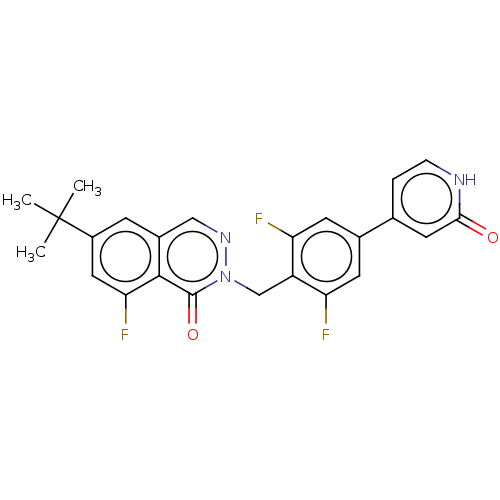 (US9458105, I-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249207 (US9458105, I-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249208 (US9458105, I-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM249209 (US9458105, I-7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.91E+3 | n/a | n/a | n/a | n/a | 7.15 | 14 |
Hoffmann-La Roche Inc. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Forster/Fluorescence Re... | US Patent US9458105 (2016) BindingDB Entry DOI: 10.7270/Q2QN65P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1592 total ) | Next | Last >> |