

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212270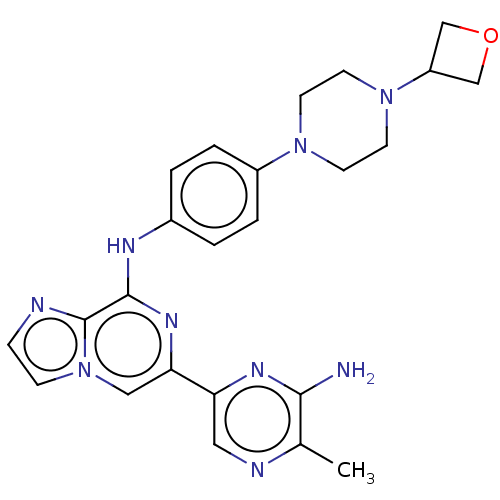 (6-(6-amino-5-methylpyrazin-2-yl)-N-(4-(4-(oxetan-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212280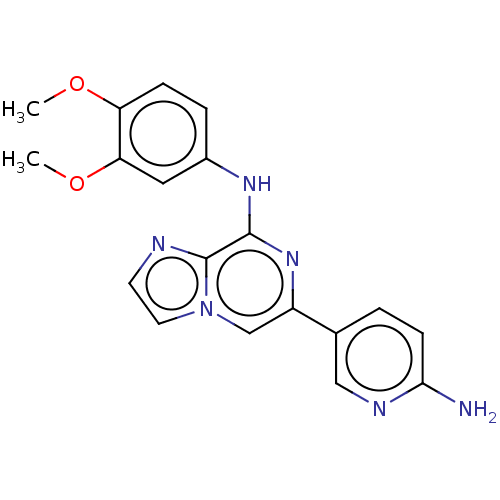 (US11517570, Example Kn.-4: | US9290505, 6-(6-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212272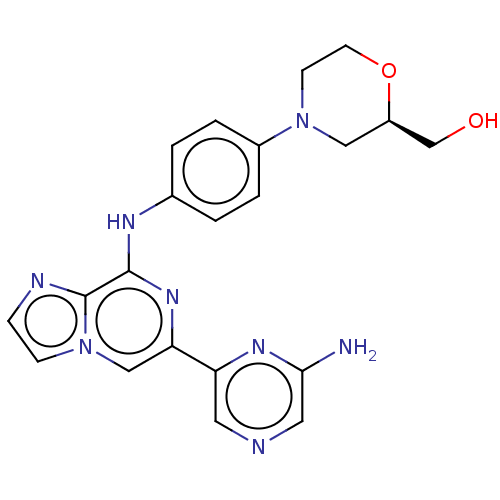 ((R)-(4-(4-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212273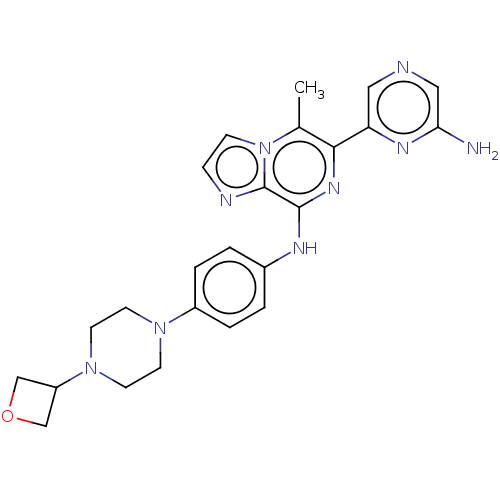 (6-(6-aminopyrazin-2-yl)-5-methyl-N-(4-(4-(oxetan-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212274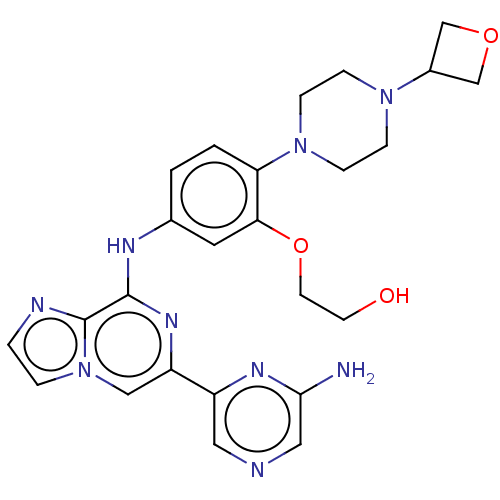 (2-(5-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]pyrazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212275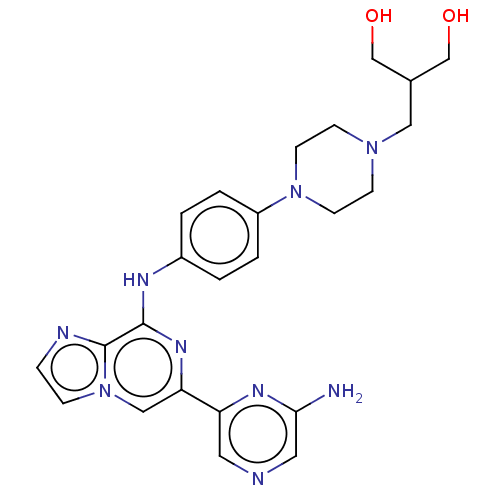 (2-((4-(4-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212276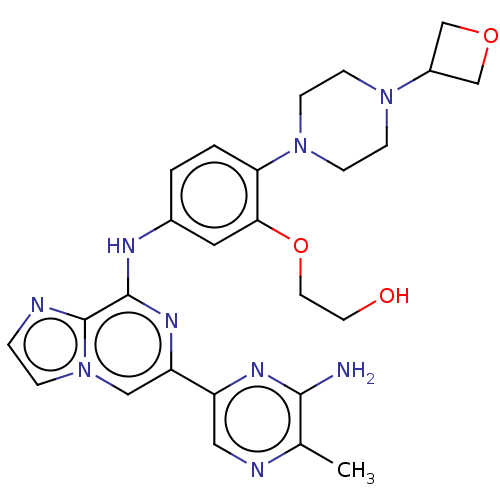 (2-(5-((6-(6-amino-5-methylpyrazin-2-yl)imidazo[1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212277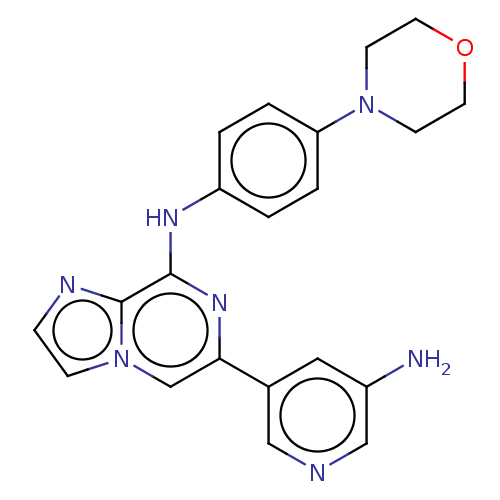 (US11517570, Example Kn.-1: | US9290505, 6-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212278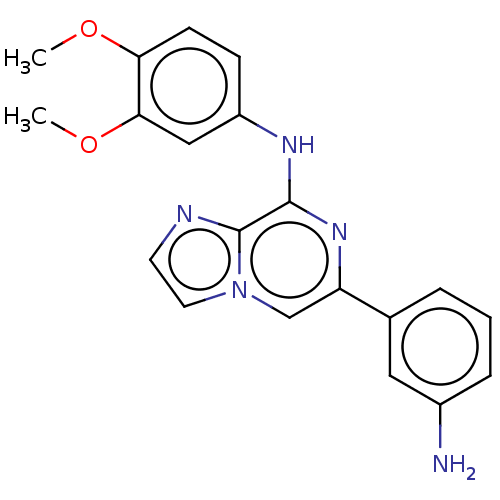 (US11517570, Example Kn.-2: | US9290505, 6-(3-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212279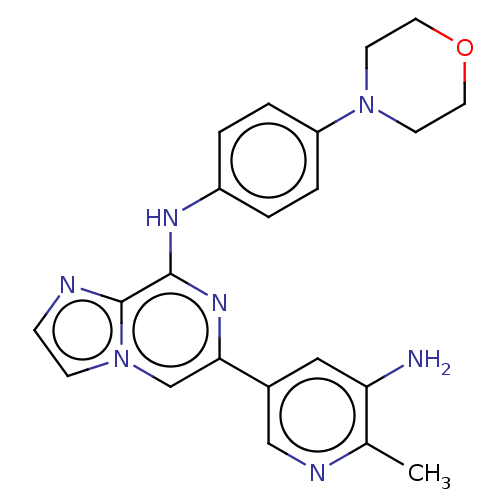 (US11517570, Example Kn.-3: | US9290505, 6-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212280 (US11517570, Example Kn.-4: | US9290505, 6-(6-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212270 (6-(6-amino-5-methylpyrazin-2-yl)-N-(4-(4-(oxetan-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212271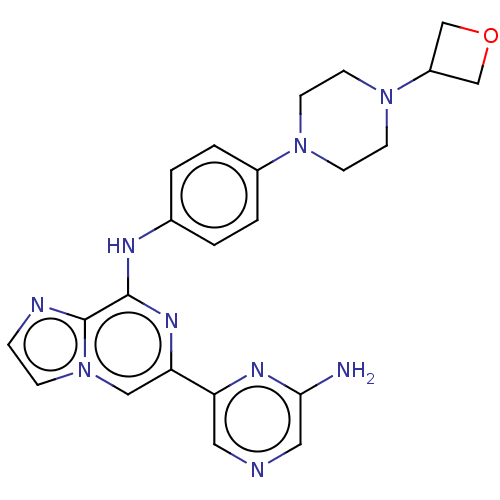 (6-(6-aminopyrazin-2-yl)-N-(4-(4-(oxetan-3-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212272 ((R)-(4-(4-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212273 (6-(6-aminopyrazin-2-yl)-5-methyl-N-(4-(4-(oxetan-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212274 (2-(5-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]pyrazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212275 (2-((4-(4-((6-(6-aminopyrazin-2-yl)imidazo[1,2-a]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212276 (2-(5-((6-(6-amino-5-methylpyrazin-2-yl)imidazo[1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212277 (US11517570, Example Kn.-1: | US9290505, 6-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212278 (US11517570, Example Kn.-2: | US9290505, 6-(3-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212279 (US11517570, Example Kn.-3: | US9290505, 6-(5-amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9504684 (2016) BindingDB Entry DOI: 10.7270/Q2XS5TB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM212271 (6-(6-aminopyrazin-2-yl)-N-(4-(4-(oxetan-3-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Syk activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, Syk-ca... | US Patent US9290505 (2016) BindingDB Entry DOI: 10.7270/Q2TB15RT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50040727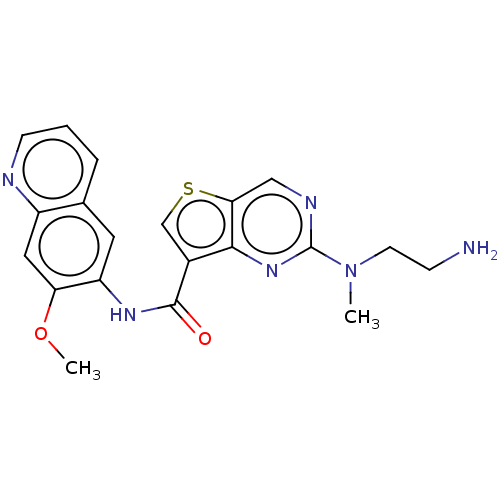 (CHEMBL3353220 | US9255110, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206177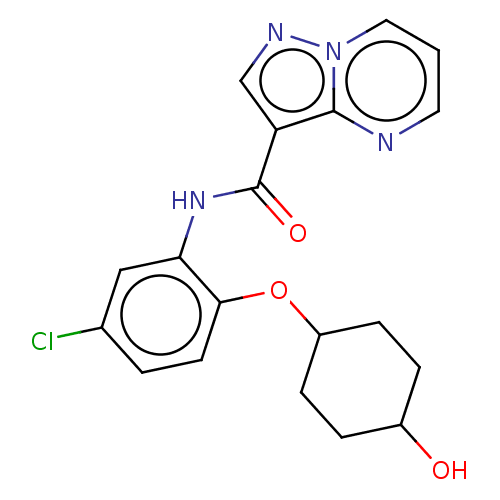 (US9255110, 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 806 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206104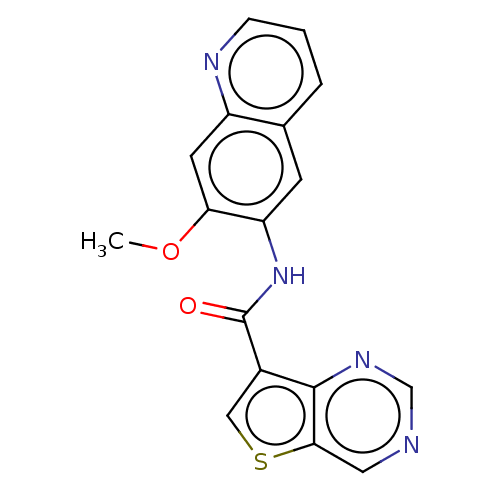 (US9255110, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 622 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206108 (US9255110, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 449 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206110 (US9255110, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 103 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206112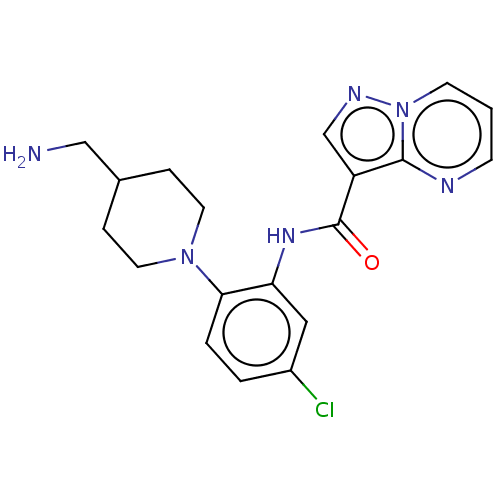 (US9255110, 25 | US9255110, 26 | US9255110, 27 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 725 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206117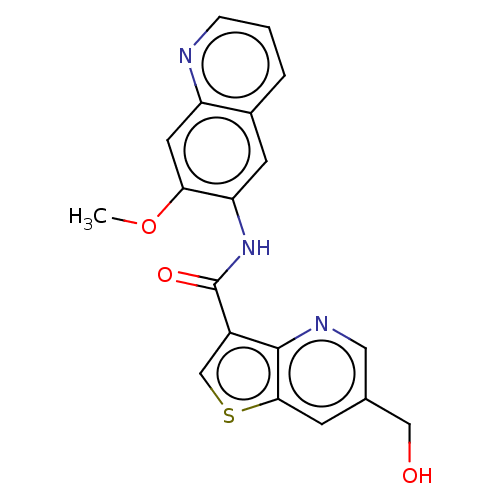 (US9255110, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 746 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206121 (US9255110, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 478 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206126 (US9255110, 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 492 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206127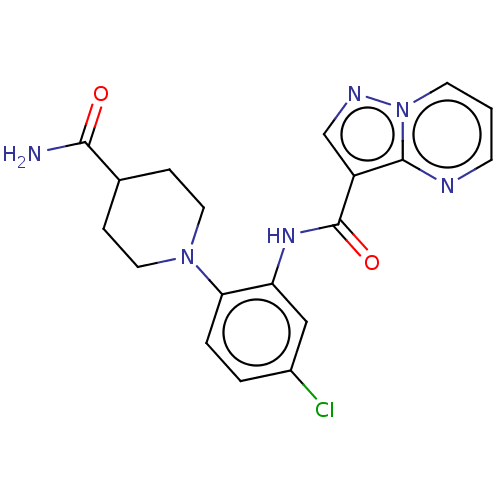 (US9255110, 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206130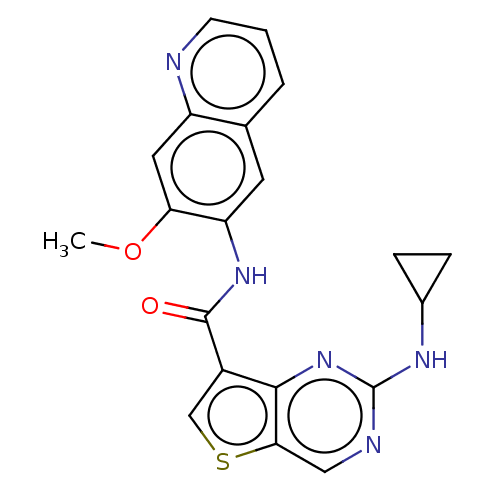 (US9255110, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206132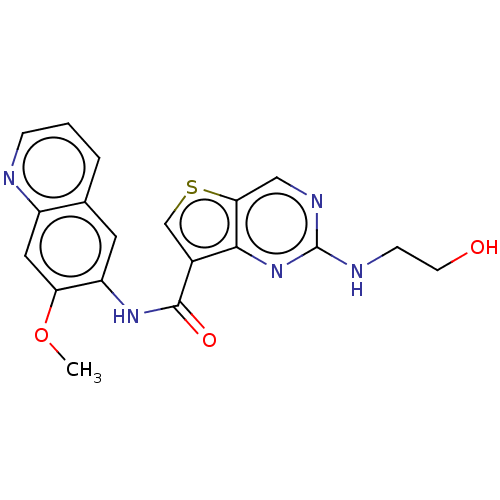 (US9255110, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 216 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206138 (US9255110, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 376 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206140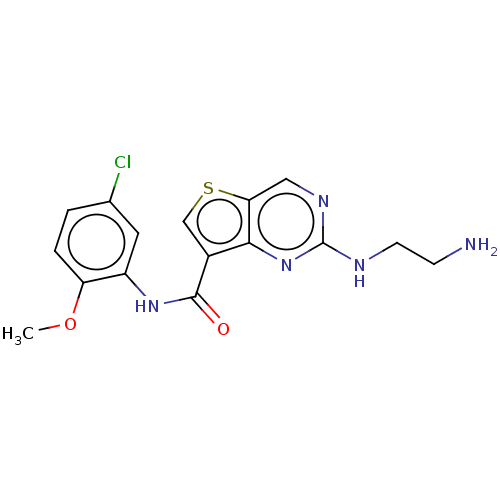 (US9255110, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206141 (US9255110, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206142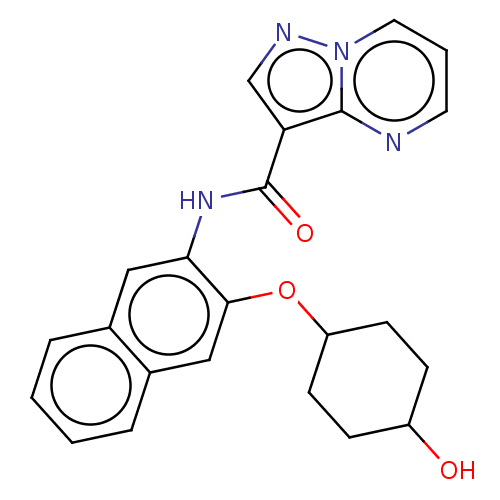 (US9255110, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 628 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206170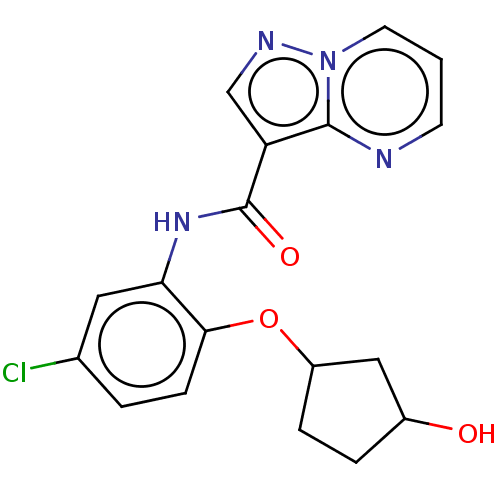 (US9255110, 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 656 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM206098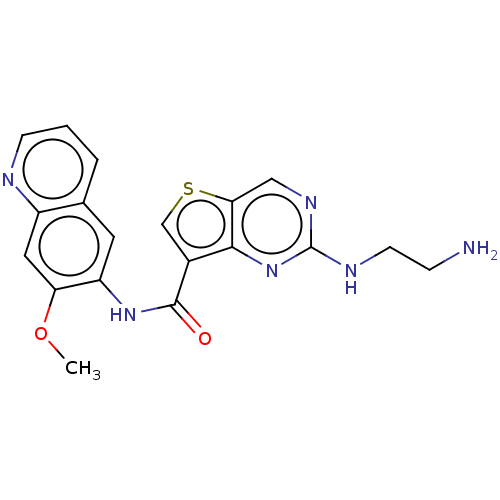 (US9255110, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.2 | 25 |
ROCHE PALO ALTO LLC; HOFFMANN-LA ROCHE INC. US Patent | Assay Description SYK activity is measured by phosphorylation of a peptide substrate (Biotin-EPEGDYEEVLE) with [gamma-33P] ATP. The enzyme reaction was conducted at 20... | US Patent US9255110 (2016) BindingDB Entry DOI: 10.7270/Q2XG9PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM255515 (US10828301, Compound R950368 | US9499493, R950368) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rigel Pharmaceuticals, Inc. US Patent | Assay Description Several 2,4-pyrimidinediamine compounds were tested for the ability to inhibit Syk kinase catalyzed phosphorylation of a peptide substrate in a bioch... | US Patent US9499493 (2016) BindingDB Entry DOI: 10.7270/Q2WW7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM255526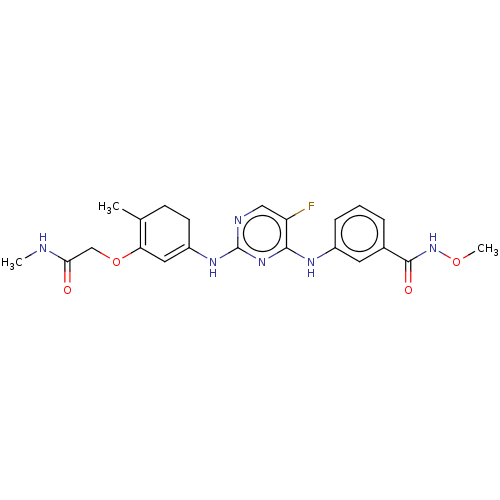 (N4-(3-Methylcarbonyloximephenyl)-5-fluoro-N2-[3-(N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rigel Pharmaceuticals, Inc. US Patent | Assay Description Several 2,4-pyrimidinediamine compounds were tested for the ability to inhibit Syk kinase catalyzed phosphorylation of a peptide substrate in a bioch... | US Patent US9499493 (2016) BindingDB Entry DOI: 10.7270/Q2WW7GKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM5057 (1,2,4-Oxadiazole Analogue 4b | 4-[(2S)-2-{[(1S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM5059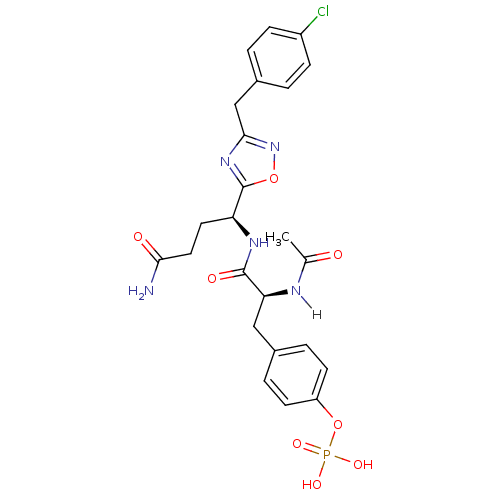 (1,2,4-Oxadiazole Analogue 4d | 4-[(2S)-2-{[(1S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM5061 (1,2,4-Oxadiazole Analogue 4g | 4-[(2S)-2-{[(1S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM5063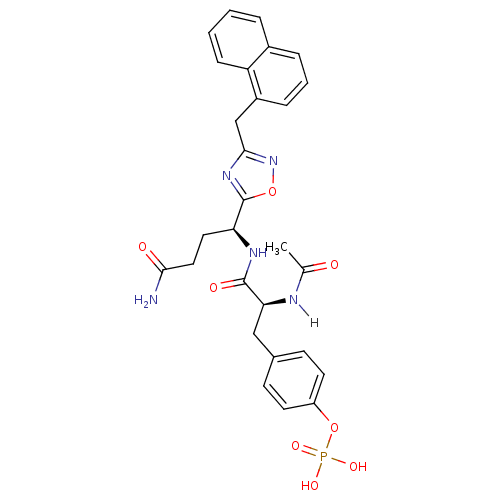 (1,2,4-Oxadiazole Analogue 4i | 4-[(2S)-2-{[(1S)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
ARIAD Pharmaceuticals, Inc. | Assay Description Fluorescence polarization competitive binding assays were used to measure the IC50s of compounds binding to the different SH2 domains. The difference... | J Med Chem 42: 4088-98 (1999) Article DOI: 10.1021/jm990229t BindingDB Entry DOI: 10.7270/Q27D2SBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM92624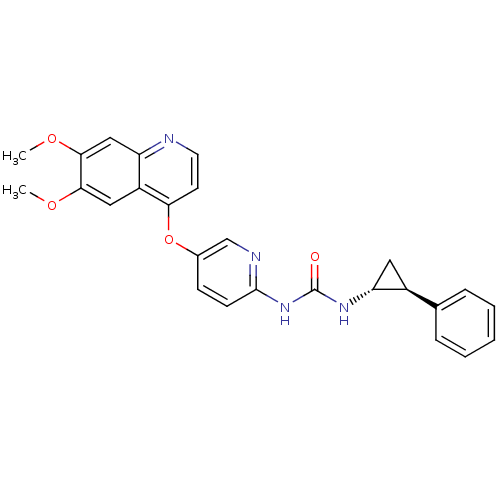 (Dimethoxyquinoline, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer | Assay Description A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain. | Chem Biol Drug Des 80: 657-64 (2012) Article DOI: 10.1111/j.1747-0285.2012.01443.x BindingDB Entry DOI: 10.7270/Q2BG2MKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM92625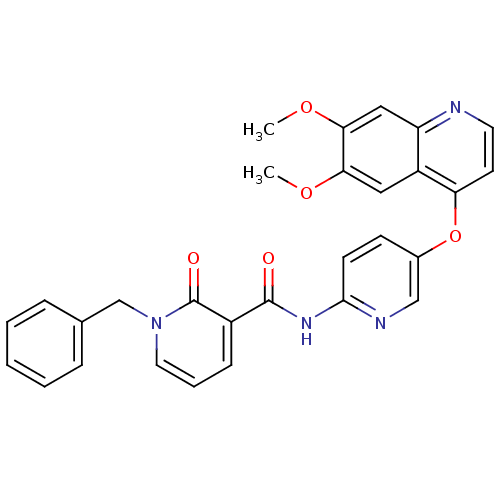 (Dimethoxyquinoline, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer | Assay Description A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain. | Chem Biol Drug Des 80: 657-64 (2012) Article DOI: 10.1111/j.1747-0285.2012.01443.x BindingDB Entry DOI: 10.7270/Q2BG2MKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM92626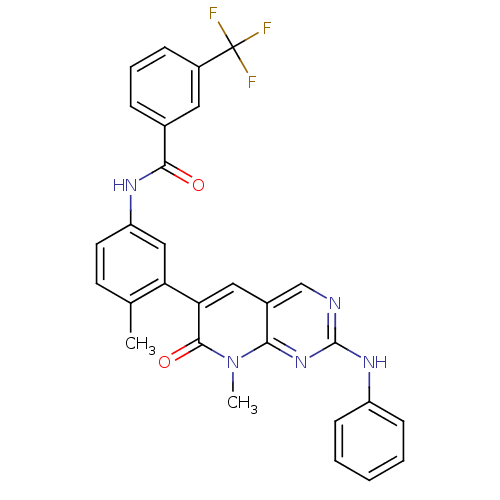 (Pyrido analog, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer | Assay Description A Lance TR-FRET assay (Perkin Elmer) was used to measure and compare the potency of compounds against Syk kinase domain. | Chem Biol Drug Des 80: 657-64 (2012) Article DOI: 10.1111/j.1747-0285.2012.01443.x BindingDB Entry DOI: 10.7270/Q2BG2MKC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50419247 (CHEMBL1835071 | US8470835, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Glaxo Group Limited US Patent | Assay Description Recombinant human Syk was express as a His-tagged protein. The activity of Syk was asses using a time-resolved fluorescence resonance entery transfe... | US Patent US8470835 (2013) BindingDB Entry DOI: 10.7270/Q28S4NJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1793 total ) | Next | Last >> |