 Found 151 hits of ph data with Target = 'Urokinase-type plasminogen activator'
Found 151 hits of ph data with Target = 'Urokinase-type plasminogen activator' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92292
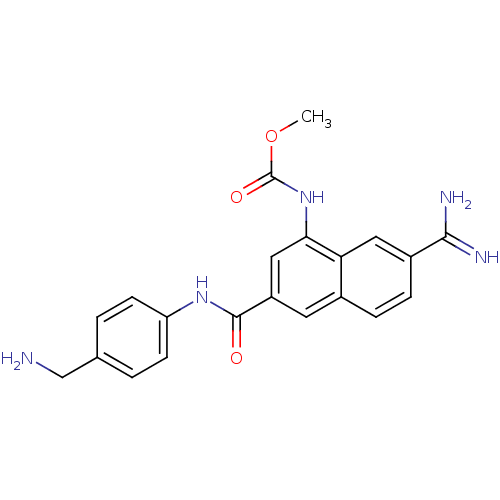
(uPa_17)Show SMILES COC(=O)Nc1cc(cc2ccc(cc12)C(N)=N)C(=O)Nc1ccc(CN)cc1 Show InChI InChI=1S/C21H21N5O3/c1-29-21(28)26-18-10-15(8-13-4-5-14(19(23)24)9-17(13)18)20(27)25-16-6-2-12(11-22)3-7-16/h2-10H,11,22H2,1H3,(H3,23,24)(H,25,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14142
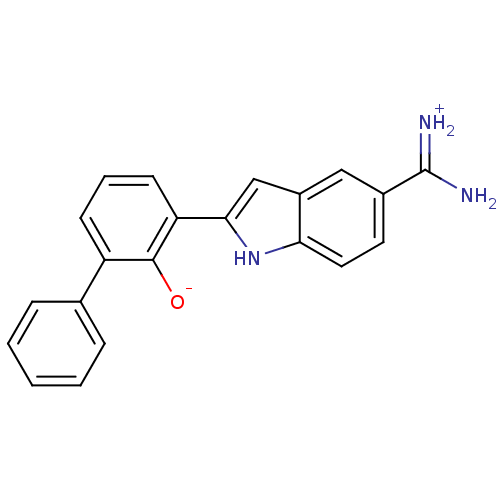
(2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92321
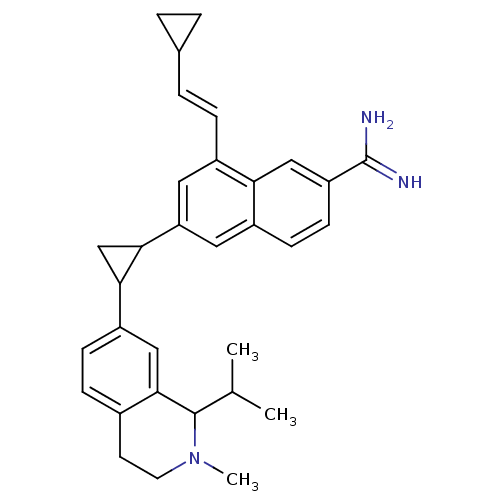
(uPa_43)Show SMILES CC(C)C1N(C)CCc2ccc(cc12)C1CC1c1cc(\C=C\C2CC2)c2cc(ccc2c1)C(N)=N Show InChI InChI=1S/C32H37N3/c1-19(2)31-30-16-24(9-8-21(30)12-13-35(31)3)28-18-29(28)26-14-22(7-6-20-4-5-20)27-17-25(32(33)34)11-10-23(27)15-26/h6-11,14-17,19-20,28-29,31H,4-5,12-13,18H2,1-3H3,(H3,33,34)/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14152
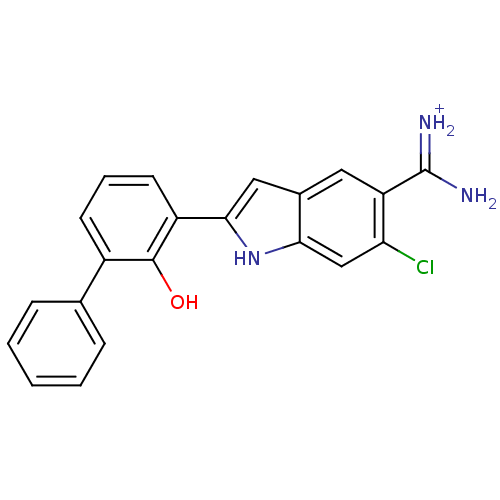
(6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14149
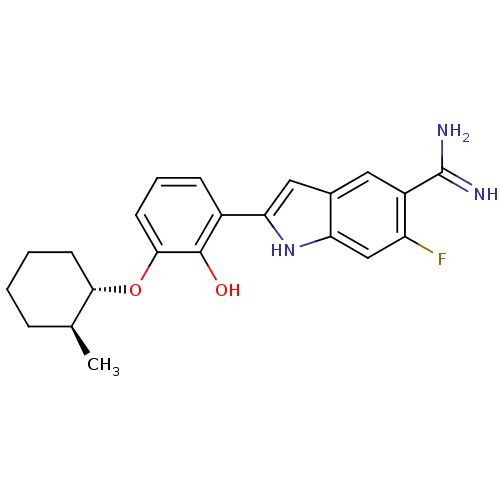
(6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2cc3cc(C(N)=N)c(F)cc3[nH]2)c1O |r| Show InChI InChI=1S/C22H24FN3O2/c1-12-5-2-3-7-19(12)28-20-8-4-6-14(21(20)27)18-10-13-9-15(22(24)25)16(23)11-17(13)26-18/h4,6,8-12,19,26-27H,2-3,5,7H2,1H3,(H3,24,25)/t12-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92302

(uPa_26)Show SMILES CC(C)C1=NCCc2ccc(NC(=O)c3cc(Br)c4cc(ccc4c3)C(N)=N)cc12 |t:3| Show InChI InChI=1S/C24H23BrN4O/c1-13(2)22-20-12-18(6-5-14(20)7-8-28-22)29-24(30)17-9-15-3-4-16(23(26)27)10-19(15)21(25)11-17/h3-6,9-13H,7-8H2,1-2H3,(H3,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92313

(uPa_36)Show SMILES COC(=O)Nc1cc(cc2ccc(cc12)C(N)=N)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H18N4O3/c1-27-20(26)24-17-11-14(19(25)23-15-5-3-2-4-6-15)9-12-7-8-13(18(21)22)10-16(12)17/h2-11H,1H3,(H3,21,22)(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14150
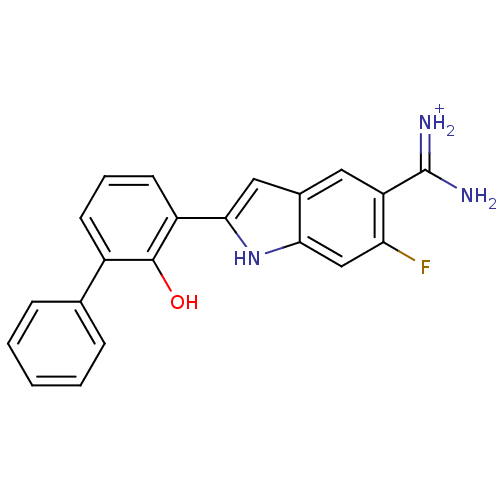
(APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1F)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16FN3O/c22-17-11-18-13(9-16(17)21(23)24)10-19(25-18)15-8-4-7-14(20(15)26)12-5-2-1-3-6-12/h1-11,25-26H,(H3,23,24)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92316
(uPa_39)Show SMILES COC\C=C\c1cc(cc2ccc(cc12)C(N)=N)C1CC1c1ccc2CCN(C)C(C(C)C)c2c1 Show InChI InChI=1S/C31H37N3O/c1-19(2)30-29-16-23(8-7-20(29)11-12-34(30)3)27-18-28(27)25-14-21(6-5-13-35-4)26-17-24(31(32)33)10-9-22(26)15-25/h5-10,14-17,19,27-28,30H,11-13,18H2,1-4H3,(H3,32,33)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92304

(uPa_28)Show SMILES COc1cc(NC(=O)c2ccc3cc(ccc3c2)C(N)=N)cc(OC(C)C)c1 Show InChI InChI=1S/C22H23N3O3/c1-13(2)28-20-11-18(10-19(12-20)27-3)25-22(26)17-7-5-14-8-16(21(23)24)6-4-15(14)9-17/h4-13H,1-3H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92287
(uPa_10)Show InChI InChI=1S/C13H13N3O2/c1-18-13(17)16-11-4-2-3-8-5-6-9(12(14)15)7-10(8)11/h2-7H,1H3,(H3,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138662
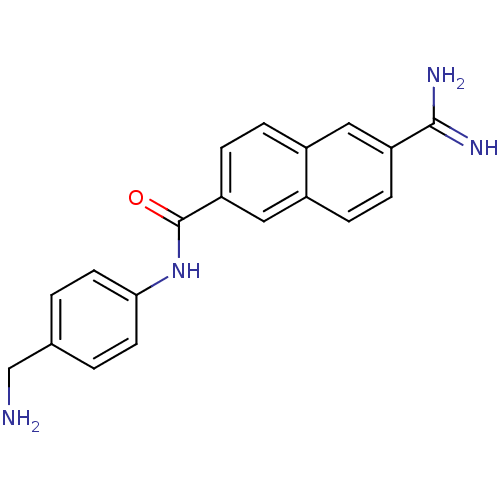
(6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-a...)Show InChI InChI=1S/C19H18N4O/c20-11-12-1-7-17(8-2-12)23-19(24)16-6-4-13-9-15(18(21)22)5-3-14(13)10-16/h1-10H,11,20H2,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92319
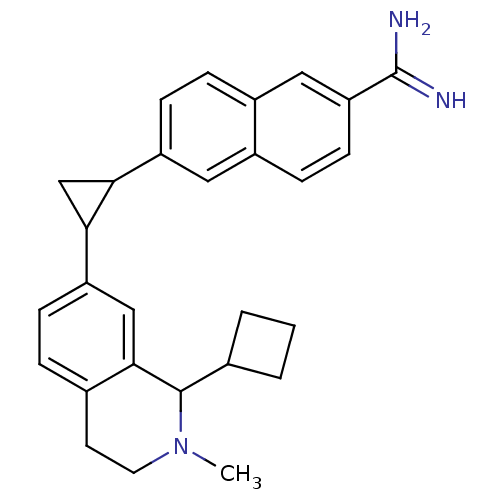
(uPa_41)Show SMILES CN1CCc2ccc(cc2C1C1CCC1)C1CC1c1ccc2cc(ccc2c1)C(N)=N Show InChI InChI=1S/C28H31N3/c1-31-12-11-17-5-8-22(15-26(17)27(31)18-3-2-4-18)25-16-24(25)21-9-6-20-14-23(28(29)30)10-7-19(20)13-21/h5-10,13-15,18,24-25,27H,2-4,11-12,16H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 42 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92293
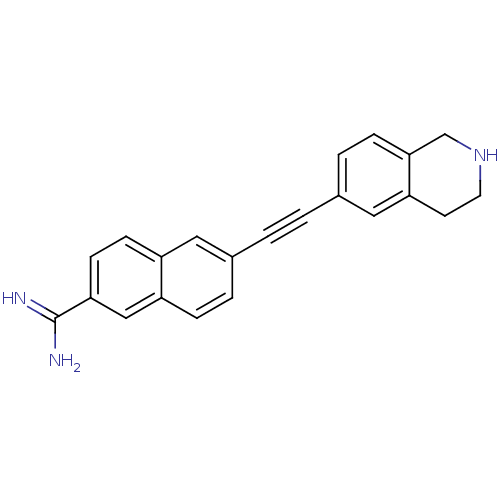
(uPa_18)Show InChI InChI=1S/C22H19N3/c23-22(24)20-8-7-17-11-15(3-5-18(17)13-20)1-2-16-4-6-21-14-25-10-9-19(21)12-16/h3-8,11-13,25H,9-10,14H2,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 59 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92308
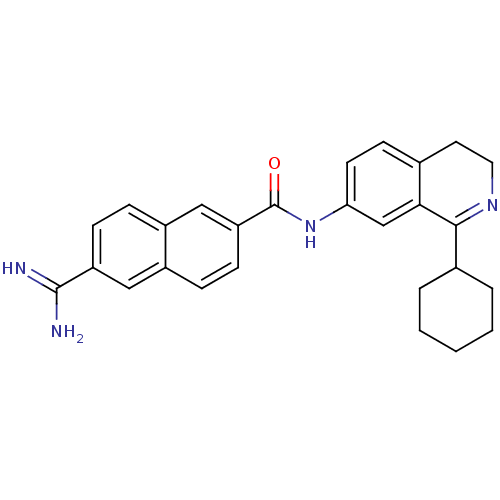
(uPa_31)Show SMILES NC(=N)c1ccc2cc(ccc2c1)C(=O)Nc1ccc2CCN=C(C3CCCCC3)c2c1 |t:24| Show InChI InChI=1S/C27H28N4O/c28-26(29)21-8-6-20-15-22(9-7-19(20)14-21)27(32)31-23-11-10-17-12-13-30-25(24(17)16-23)18-4-2-1-3-5-18/h6-11,14-16,18H,1-5,12-13H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 66 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14156
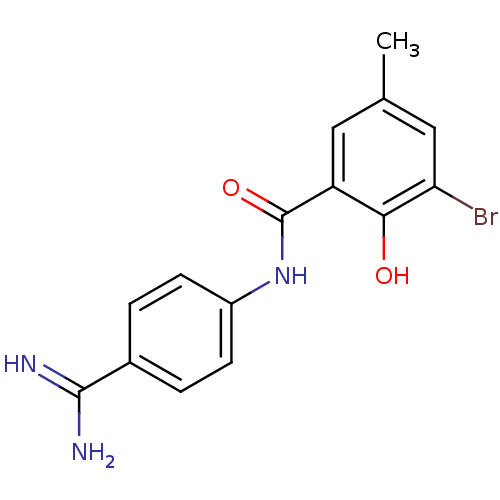
(3-bromo-N-(4-carbamimidoylphenyl)-2-hydroxy-5-meth...)Show InChI InChI=1S/C15H14BrN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92320

(uPa_42)Show SMILES COc1cc(cc2ccc(cc12)C(N)=N)C1CC1c1ccc2CCN(C)C(C(C)C)c2c1 Show InChI InChI=1S/C28H33N3O/c1-16(2)27-25-12-19(6-5-17(25)9-10-31(27)3)22-15-23(22)21-11-18-7-8-20(28(29)30)13-24(18)26(14-21)32-4/h5-8,11-14,16,22-23,27H,9-10,15H2,1-4H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 94 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14148
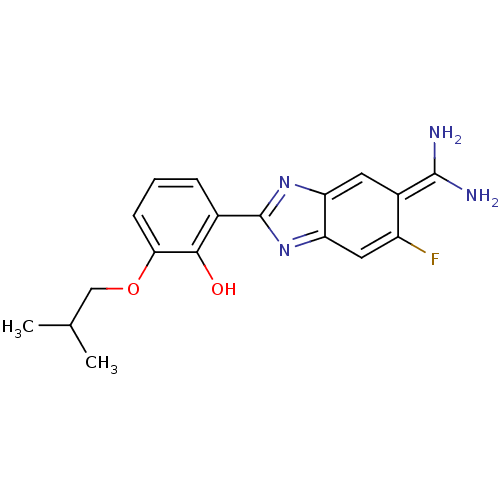
(2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...)Show SMILES CC(C)COc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=[NH2+])c1[O-] Show InChI InChI=1S/C18H19FN4O2/c1-9(2)8-25-15-5-3-4-10(16(15)24)18-22-13-6-11(17(20)21)12(19)7-14(13)23-18/h3-7,9,24H,8H2,1-2H3,(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 110 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14146

(APC-9008 | {amino[2-(3,5-dichloro-2-hydroxyphenyl)...)Show SMILES NC(=[NH2+])c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(Cl)c1O Show InChI InChI=1S/C15H11Cl2N3O/c16-9-5-10(14(21)11(17)6-9)13-4-8-3-7(15(18)19)1-2-12(8)20-13/h1-6,20-21H,(H3,18,19)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14169
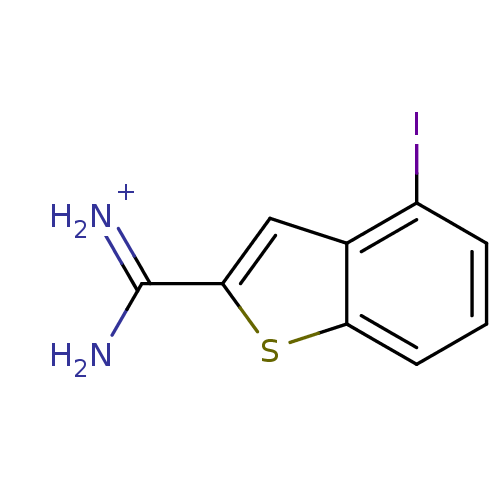
(4-Iodobenzo[b]thiophene-2-carboxamidine | APC-6860...)Show InChI InChI=1S/C9H7IN2S/c10-6-2-1-3-7-5(6)4-8(13-7)9(11)12/h1-4H,(H3,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 210 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 7: 299-312 (2000)
Article DOI: 10.1016/S1074-5521(00)00104-6
BindingDB Entry DOI: 10.7270/Q2NG4NWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14144
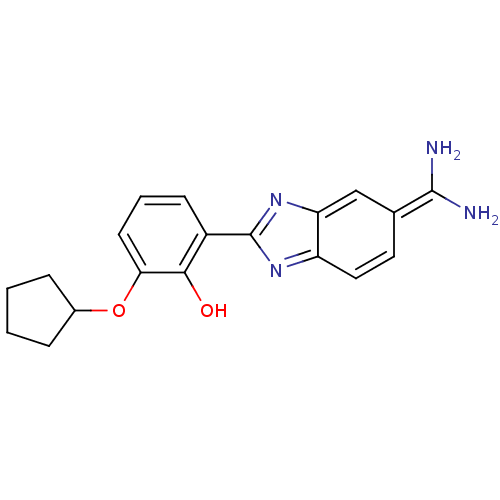
(2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...)Show SMILES NC(=[NH2+])c1ccc2nc([nH]c2c1)-c1cccc(OC2CCCC2)c1[O-] Show InChI InChI=1S/C19H20N4O2/c20-18(21)11-8-9-14-15(10-11)23-19(22-14)13-6-3-7-16(17(13)24)25-12-4-1-2-5-12/h3,6-10,12,24H,1-2,4-5H2,(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 220 | -37.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92318
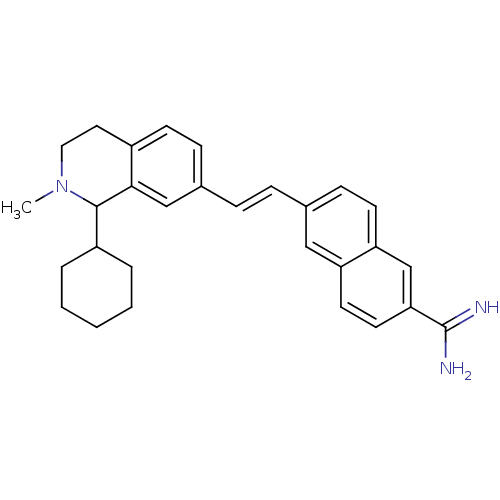
(uPa_40)Show SMILES CN1CCc2ccc(\C=C\c3ccc4cc(ccc4c3)C(N)=N)cc2C1C1CCCCC1 Show InChI InChI=1S/C29H33N3/c1-32-16-15-22-11-9-21(18-27(22)28(32)23-5-3-2-4-6-23)8-7-20-10-12-25-19-26(29(30)31)14-13-24(25)17-20/h7-14,17-19,23,28H,2-6,15-16H2,1H3,(H3,30,31)/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 223 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14158
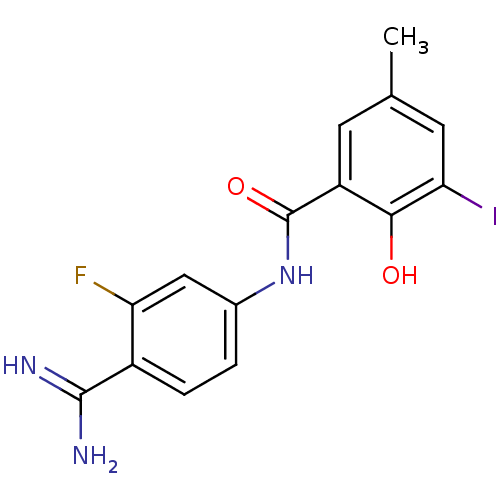
(APC-11922 | CHEMBL293260 | N-(4-carbamimidoyl-3-fl...)Show InChI InChI=1S/C15H13FIN3O2/c1-7-4-10(13(21)12(17)5-7)15(22)20-8-2-3-9(14(18)19)11(16)6-8/h2-6,21H,1H3,(H3,18,19)(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92305
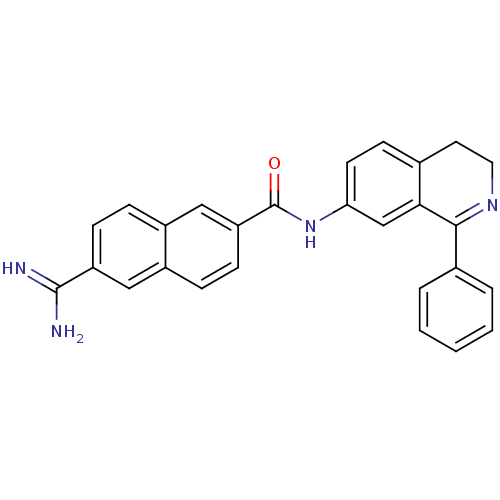
(uPa_29)Show SMILES NC(=N)c1ccc2cc(ccc2c1)C(=O)Nc1ccc2CCN=C(c3ccccc3)c2c1 |t:24| Show InChI InChI=1S/C27H22N4O/c28-26(29)21-8-6-20-15-22(9-7-19(20)14-21)27(32)31-23-11-10-17-12-13-30-25(24(17)16-23)18-4-2-1-3-5-18/h1-11,14-16H,12-13H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 290 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14151
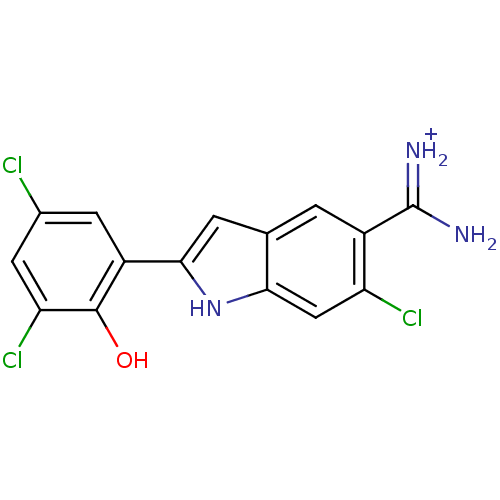
(APC-9850 | {amino[6-chloro-2-(3,5-dichloro-2-hydro...)Show SMILES NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cc(Cl)cc(Cl)c1O Show InChI InChI=1S/C15H10Cl3N3O/c16-7-3-9(14(22)11(18)4-7)13-2-6-1-8(15(19)20)10(17)5-12(6)21-13/h1-5,21-22H,(H3,19,20)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14157

(APC-10605 | CHEMBL64097 | N-(4-carbamimidoylphenyl...)Show InChI InChI=1S/C15H14IN3O2/c1-8-6-11(13(20)12(16)7-8)15(21)19-10-4-2-9(3-5-10)14(17)18/h2-7,20H,1H3,(H3,17,18)(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92294

(uPa_19)Show InChI InChI=1S/C21H20N2O/c22-21(23)20-9-8-18-13-17(6-7-19(18)14-20)5-4-15-2-1-3-16(12-15)10-11-24/h1-9,12-14,24H,10-11H2,(H3,22,23)/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 406 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147092
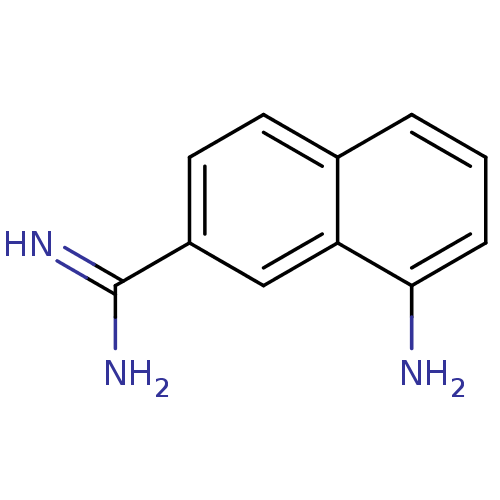
(8-Amino-naphthalene-2-carboxamidine | 8-amino-2-na...)Show InChI InChI=1S/C11H11N3/c12-10-3-1-2-7-4-5-8(11(13)14)6-9(7)10/h1-6H,12H2,(H3,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| 450 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14143
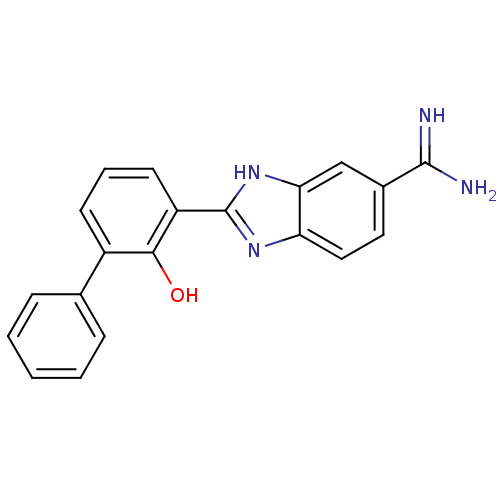
(2-(2-HYDROXY-BIPHENYL)-1H-BENZOIMIDAZOLE-5-CARBOXA...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C20H16N4O/c21-19(22)13-9-10-16-17(11-13)24-20(23-16)15-8-4-7-14(18(15)25)12-5-2-1-3-6-12/h1-11,25H,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 450 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14145

(2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...)Show SMILES CC(C)COc1cccc(-c2nc3ccc(cc3[nH]2)C(N)=[NH2+])c1[O-] Show InChI InChI=1S/C18H20N4O2/c1-10(2)9-24-15-5-3-4-12(16(15)23)18-21-13-7-6-11(17(19)20)8-14(13)22-18/h3-8,10,23H,9H2,1-2H3,(H3,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 500 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14147
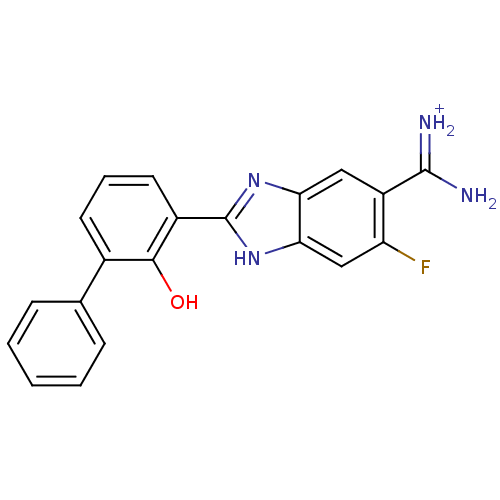
(APC-10818 | {amino[6-fluoro-2-(2-hydroxy-3-phenylp...)Show SMILES NC(=[NH2+])c1cc2nc([nH]c2cc1F)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C20H15FN4O/c21-15-10-17-16(9-14(15)19(22)23)24-20(25-17)13-8-4-7-12(18(13)26)11-5-2-1-3-6-11/h1-10,26H,(H3,22,23)(H,24,25)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | -35.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92289
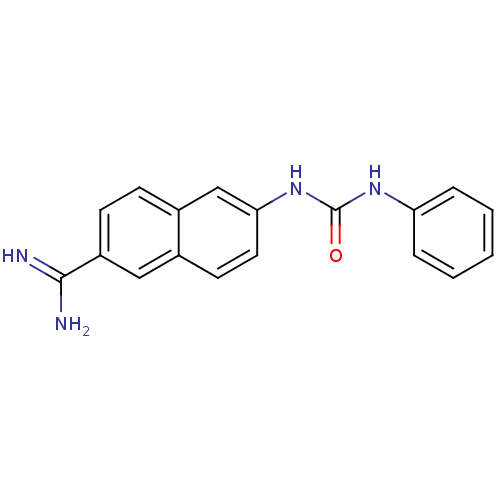
(uPa_12)Show InChI InChI=1S/C18H16N4O/c19-17(20)14-7-6-13-11-16(9-8-12(13)10-14)22-18(23)21-15-4-2-1-3-5-15/h1-11H,(H3,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 610 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92325
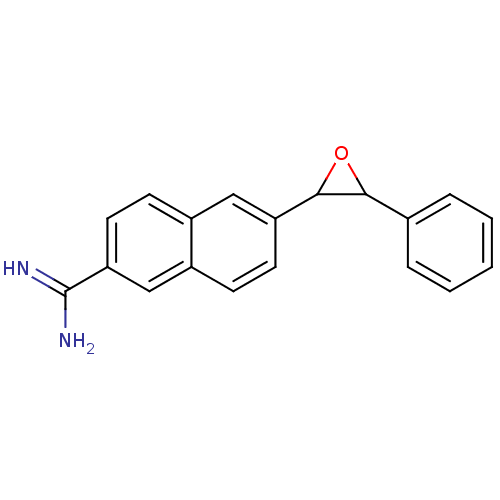
(uPa_8)Show InChI InChI=1S/C19H16N2O/c20-19(21)16-9-7-13-10-15(8-6-14(13)11-16)18-17(22-18)12-4-2-1-3-5-12/h1-11,17-18H,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 610 | -35.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138670

(6-Carbamimidoyl-naphthalene-2-carboxylic acid phen...)Show InChI InChI=1S/C18H15N3O/c19-17(20)14-8-6-13-11-15(9-7-12(13)10-14)18(22)21-16-4-2-1-3-5-16/h1-11H,(H3,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 628 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147085
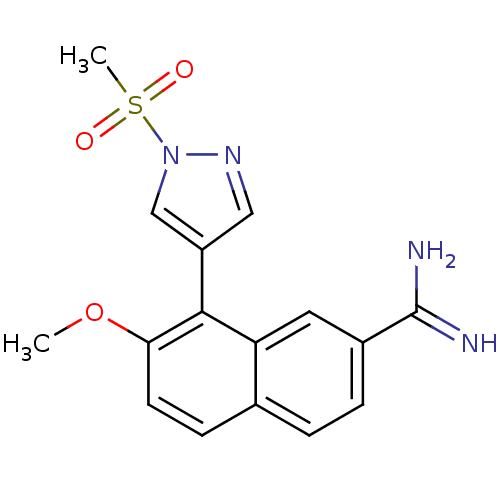
(7-METHOXY-8-[1-(METHYLSULFONYL)-1H-PYRAZOL-4-YL]NA...)Show SMILES COc1ccc2ccc(cc2c1-c1cnn(c1)S(C)(=O)=O)C(N)=N Show InChI InChI=1S/C16H16N4O3S/c1-23-14-6-5-10-3-4-11(16(17)18)7-13(10)15(14)12-8-19-20(9-12)24(2,21)22/h3-9H,1-2H3,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| 630 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92317

(uPa_4)Show InChI InChI=1S/C14H15N3O3/c1-19-11-5-4-8-2-3-9(14(16)17)6-10(8)13(11)20-7-12(15)18/h2-6H,7H2,1H3,(H2,15,18)(H3,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 637 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92301
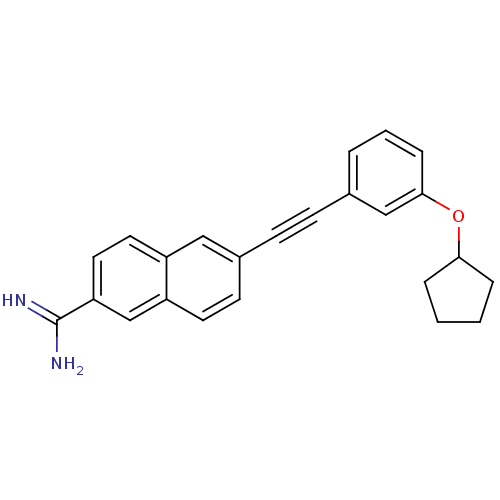
(uPa_25)Show SMILES NC(=N)c1ccc2cc(ccc2c1)C#Cc1cccc(OC2CCCC2)c1 Show InChI InChI=1S/C24H22N2O/c25-24(26)21-13-12-19-14-18(10-11-20(19)16-21)9-8-17-4-3-7-23(15-17)27-22-5-1-2-6-22/h3-4,7,10-16,22H,1-2,5-6H2,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 665 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92296

(uPa_20)Show SMILES CS(=O)(=O)n1cc(cn1)-c1cc(cc2ccc(cc12)C(N)=N)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H19N5O3S/c1-31(29,30)27-13-17(12-25-27)20-11-16(22(28)26-18-5-3-2-4-6-18)9-14-7-8-15(21(23)24)10-19(14)20/h2-13H,1H3,(H3,23,24)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 769 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92315
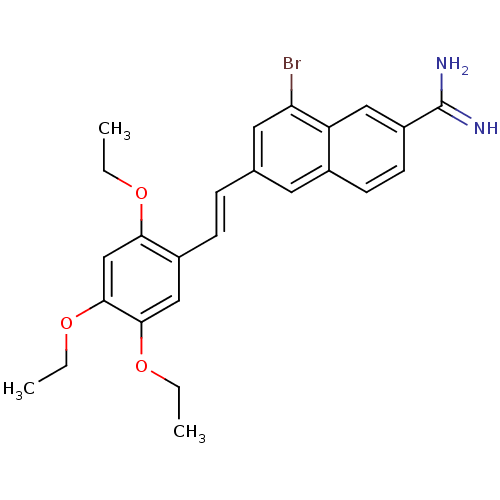
(uPa_38)Show SMILES CCOc1cc(OCC)c(\C=C\c2cc(Br)c3cc(ccc3c2)C(N)=N)cc1OCC Show InChI InChI=1S/C25H27BrN2O3/c1-4-29-22-15-24(31-6-3)23(30-5-2)14-18(22)8-7-16-11-17-9-10-19(25(27)28)13-20(17)21(26)12-16/h7-15H,4-6H2,1-3H3,(H3,27,28)/b8-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 777 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92322

(uPa_44)Show SMILES CN1CCc2ccc(cc2C1c1cccnc1)C1CC1c1ccc2cc(ccc2c1)C(N)=N Show InChI InChI=1S/C29H28N4/c1-33-12-10-18-4-7-22(15-27(18)28(33)24-3-2-11-32-17-24)26-16-25(26)21-8-5-20-14-23(29(30)31)9-6-19(20)13-21/h2-9,11,13-15,17,25-26,28H,10,12,16H2,1H3,(H3,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92314

(uPa_37)Show SMILES CS(=O)(=O)Nc1cc(CCc2ccccc2)cc2ccc(cc12)C(N)=N Show InChI InChI=1S/C20H21N3O2S/c1-26(24,25)23-19-12-15(8-7-14-5-3-2-4-6-14)11-16-9-10-17(20(21)22)13-18(16)19/h2-6,9-13,23H,7-8H2,1H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.49E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14154

(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-1,3-benz...)Show SMILES CC(C)COc1cccc(-c2nc3cc(Cl)c(cc3[nH]2)C(N)=[NH2+])c1[O-] Show InChI InChI=1S/C18H19ClN4O2/c1-9(2)8-25-15-5-3-4-10(16(15)24)18-22-13-6-11(17(20)21)12(19)7-14(13)23-18/h3-7,9,24H,8H2,1-2H3,(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.80E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14153
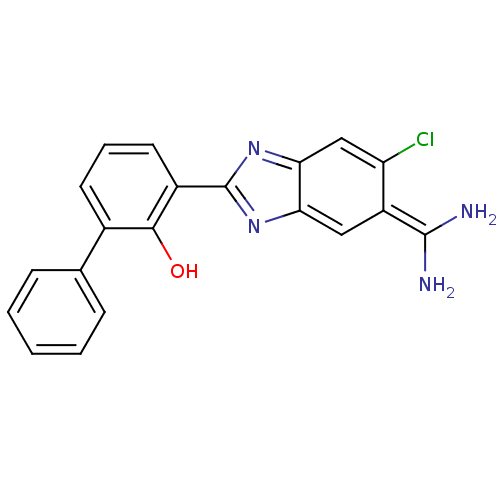
(2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-1,3-benz...)Show SMILES NC(=[NH2+])c1cc2[nH]c(nc2cc1Cl)-c1cccc(-c2ccccc2)c1[O-] Show InChI InChI=1S/C20H15ClN4O/c21-15-10-17-16(9-14(15)19(22)23)24-20(25-17)13-8-4-7-12(18(13)26)11-5-2-1-3-6-11/h1-10,26H,(H3,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.10E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14170
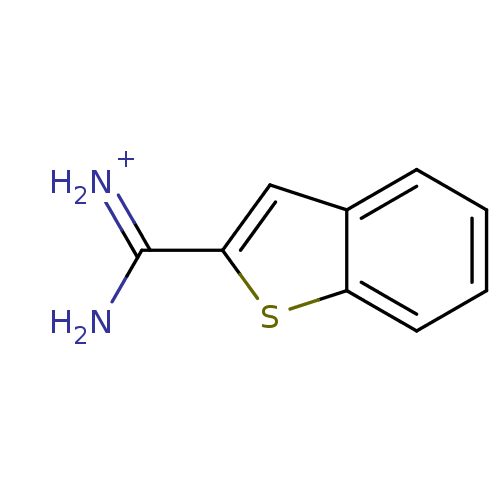
(APC-7377 | Benzo[b]thiophene-2-carboxamidine | [am...)Show InChI InChI=1S/C9H8N2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5H,(H3,10,11)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30E+3 | -31.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 7: 299-312 (2000)
Article DOI: 10.1016/S1074-5521(00)00104-6
BindingDB Entry DOI: 10.7270/Q2NG4NWC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92309

(uPa_32)Show InChI InChI=1S/C20H18BrN3/c21-19-11-15(5-2-1-4-14-6-3-9-24-13-14)10-16-7-8-17(20(22)23)12-18(16)19/h2-3,5-13H,1,4H2,(H3,22,23)/b5-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 3.08E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92298
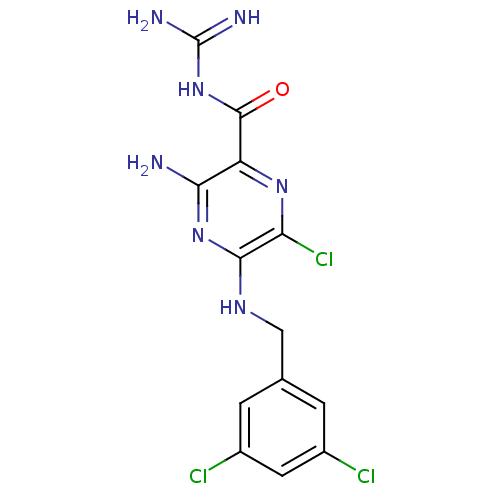
(uPa_22)Show SMILES NC(=N)NC(=O)c1nc(Cl)c(NCc2cc(Cl)cc(Cl)c2)nc1N Show InChI InChI=1S/C13H12Cl3N7O/c14-6-1-5(2-7(15)3-6)4-20-11-9(16)21-8(10(17)22-11)12(24)23-13(18)19/h1-3H,4H2,(H3,17,20,22)(H4,18,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.57E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14155

(APC-7136 | CHEMBL64708 | N-(4-CARBAMIMIDOYL-PHENYL...)Show InChI InChI=1S/C14H13N3O2/c15-13(16)9-5-7-10(8-6-9)17-14(19)11-3-1-2-4-12(11)18/h1-8,18H,(H3,15,16)(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.80E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM92288

(uPa_11)Show InChI InChI=1S/C18H20N4O/c1-11(2)22-10-14(9-21-22)17-15-8-13(18(19)20)5-4-12(15)6-7-16(17)23-3/h4-11H,1-3H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138663

(2-naphthimidamide | CHEMBL105171 | Naphthalene-2-c...)Show InChI InChI=1S/C11H10N2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H3,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
PubMed
| 5.91E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
CSAR
| Assay Description
Abbott uPA__Urokinase Human - Ki(uM) |
CSAR 1: (2012)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14159

(APC-11421 | CHEMBL62897 | N-(4-carbamimidoyl-3-chl...)Show SMILES Cc1cc(I)c(O)c(c1)C(=O)Nc1ccc(C(N)=N)c(Cl)c1 Show InChI InChI=1S/C15H13ClIN3O2/c1-7-4-10(13(21)12(17)5-7)15(22)20-8-2-3-9(14(18)19)11(16)6-8/h2-6,21H,1H3,(H3,18,19)(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 6.00E+3 | -29.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 8: 1107-21 (2001)
Article DOI: 10.1016/S1074-5521(01)00084-9
BindingDB Entry DOI: 10.7270/Q2S75DKN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data