

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50072225 ((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Effective concentration required for stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193789 (US9670209, Compound 307 1-((R)-3-(3-(Cyclopropylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416734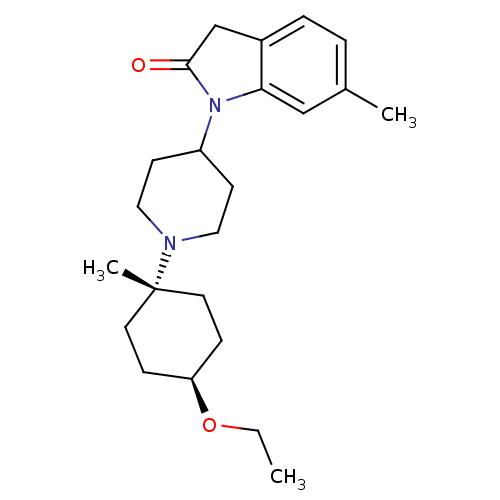 (CHEMBL1223804) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416732 (CHEMBL1223754) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193791 (US9670209, Compound 309 1-((R)-3-(3-(2-Methoxyethy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193802 (US9670209, Compound 312 1-((R)-3-(3-(cyclopropylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50072227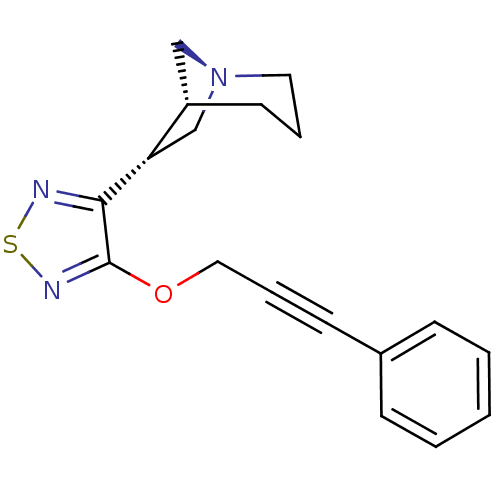 ((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416733 (CHEMBL1223803) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50034656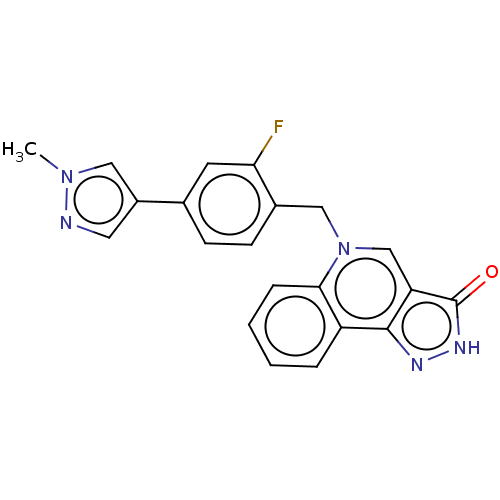 (CHEMBL3360954) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human muscarinic receptor subtype 1 expressed in CHO-K1 cells by calcium mobilization assay | Bioorg Med Chem Lett 25: 384-8 (2014) Article DOI: 10.1016/j.bmcl.2014.11.011 BindingDB Entry DOI: 10.7270/Q24X59D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263892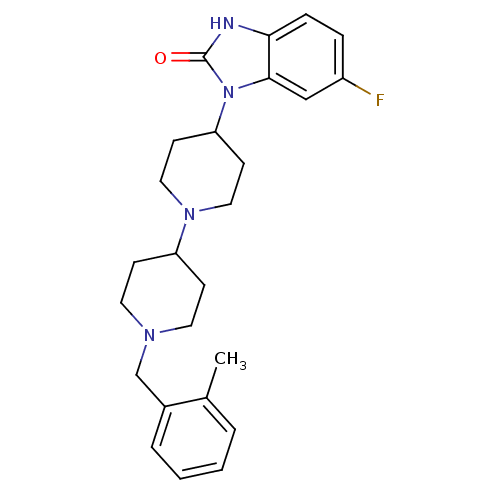 (6-Fluoro-1-[1'-(2-methyl-benzyl)-[1,4']bipiperidin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193825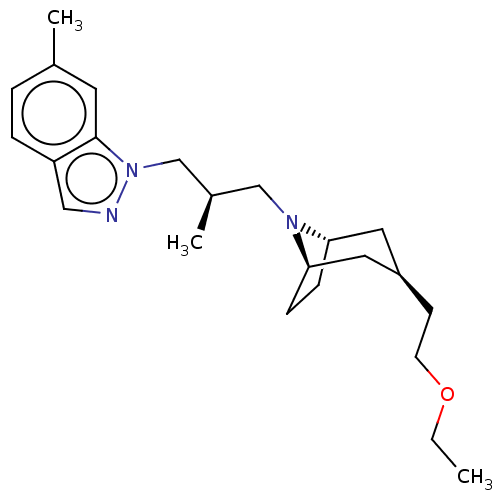 (US9670209, Compound 324 1-((R)-3-((1R,3R,5S)-3-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193823 (US9670209, Compound 323 1-((R)-3-((1R,3R,5S)-3-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416141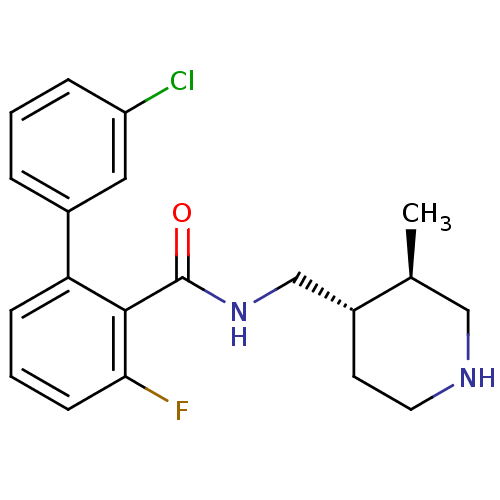 (CHEMBL1083925) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on acetylcholine-induced intracellular calcium level by FL... | Bioorg Med Chem Lett 20: 3545-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.127 BindingDB Entry DOI: 10.7270/Q2QF8V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263891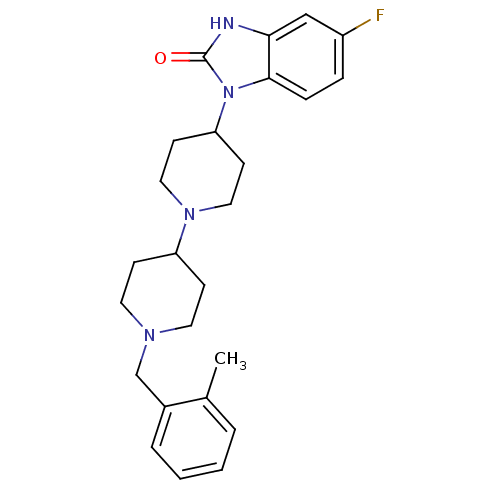 (5-Fluoro-1-[1'-(2-methyl-benzyl)-[1,4']bipiperidin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50072214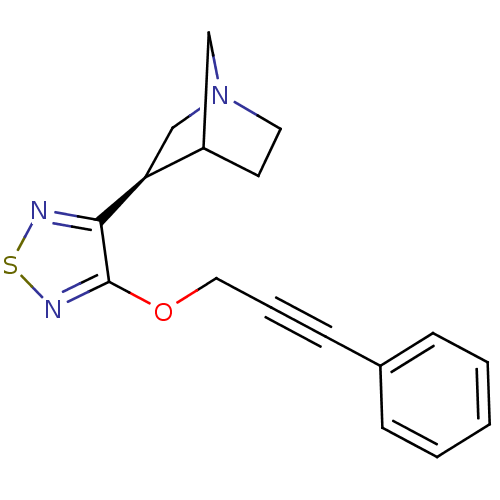 ((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50072224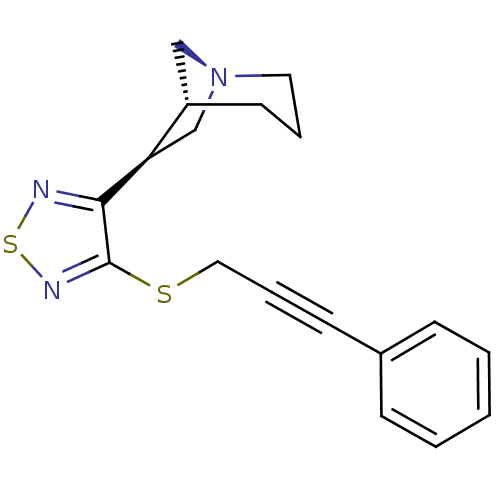 ((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor | Bioorg Med Chem Lett 8: 2897-902 (1999) BindingDB Entry DOI: 10.7270/Q27M08GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193834 (US9670209, Comparative compound 2) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50062577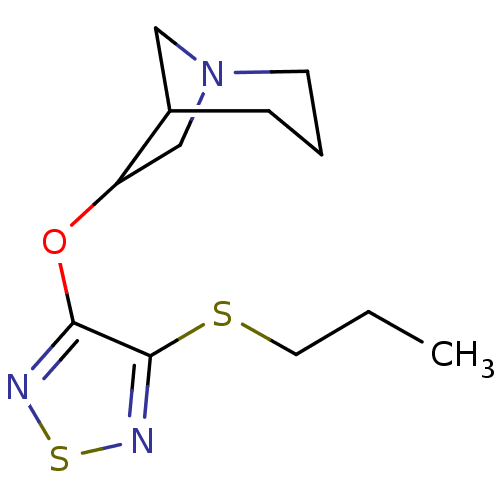 (6-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Effective concentration required for stimulation of phosphoinositol (PI) hydrolysis in the A9 L cell line transfected with the Muscarinic acetylcholi... | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416738 (CHEMBL1223860) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416151 (CHEMBL1084850) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on acetylcholine-induced intracellular calcium level by FL... | Bioorg Med Chem Lett 20: 3545-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.127 BindingDB Entry DOI: 10.7270/Q2QF8V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263936 (5-Fluoro-1-[1'-(2-trifluoromethyl-benzyl)-[1,4']bi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263890 (4-Fluoro-1-[1'-(2-methyl-benzyl)-[1,4']bipiperidin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO-K1 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assay | J Med Chem 53: 6386-97 (2010) Article DOI: 10.1021/jm100697g BindingDB Entry DOI: 10.7270/Q2765G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human M1 receptor expressed in CHO-K1 cells assessed as calcium mobilization for 6 mins by Calcium4-based staining | Bioorg Med Chem Lett 22: 5134-40 (2012) Article DOI: 10.1016/j.bmcl.2012.05.048 BindingDB Entry DOI: 10.7270/Q24M95MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50079583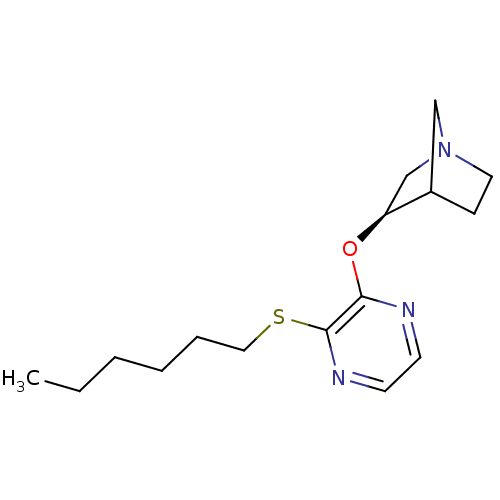 ((R)-3-(3-Hexylsulfanyl-pyrazin-2-yloxy)-1-aza-bicy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro functional agonism against M1 muscarinic receptor (PI) | Bioorg Med Chem Lett 9: 1895-900 (1999) BindingDB Entry DOI: 10.7270/Q2G161CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50034655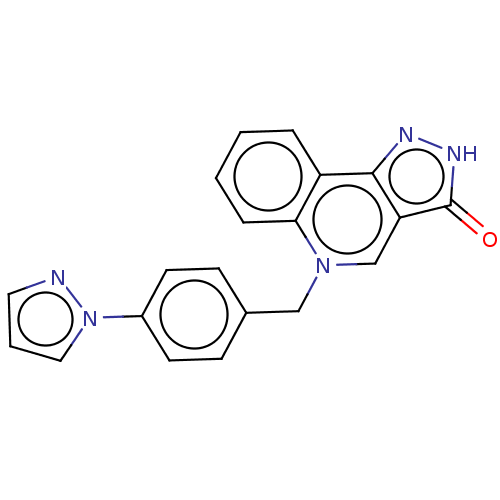 (CHEMBL3360955) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human muscarinic receptor subtype 1 expressed in CHO-K1 cells by calcium mobilization assay | Bioorg Med Chem Lett 25: 384-8 (2014) Article DOI: 10.1016/j.bmcl.2014.11.011 BindingDB Entry DOI: 10.7270/Q24X59D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416739 (CHEMBL1223861) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193790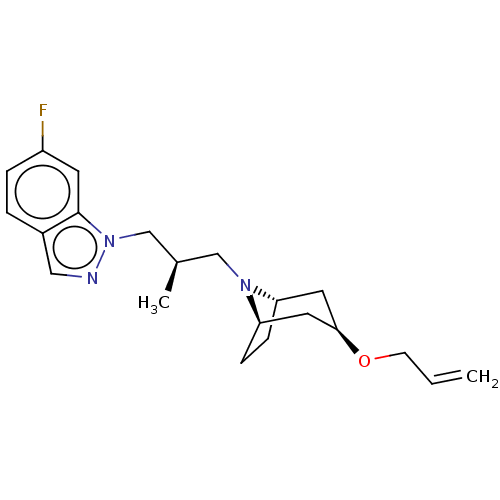 (US9670209, Compound 308 1-((R)-3-(3-(Allyloxy)-8-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193787 (US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193784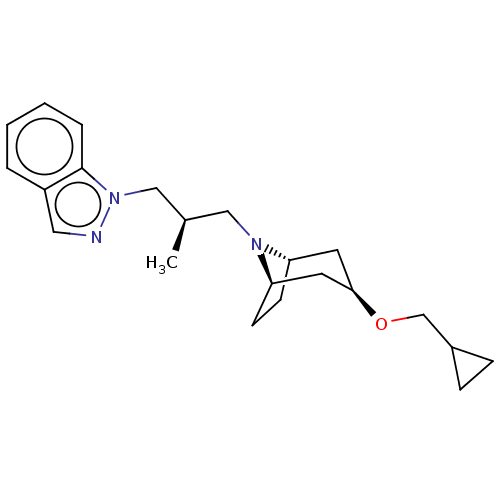 (US9670209, Compound 302 1-((R)-3-((1R,3R,5S)-3-(cy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193787 (US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO-K1 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assay | J Med Chem 53: 6386-97 (2010) Article DOI: 10.1021/jm100697g BindingDB Entry DOI: 10.7270/Q2765G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Agonist activity at human m1 muscarinic acetylcholine receptor expressed in HEK293 cells assessed as calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 25: 1546-51 (2015) Article DOI: 10.1016/j.bmcl.2015.02.012 BindingDB Entry DOI: 10.7270/Q2V69M8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263893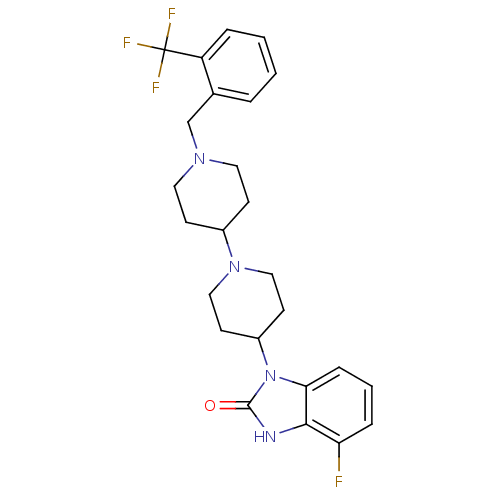 (4-Fluoro-1-[1'-(2-trifluoromethyl-benzyl)-[1,4']bi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.71 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50492269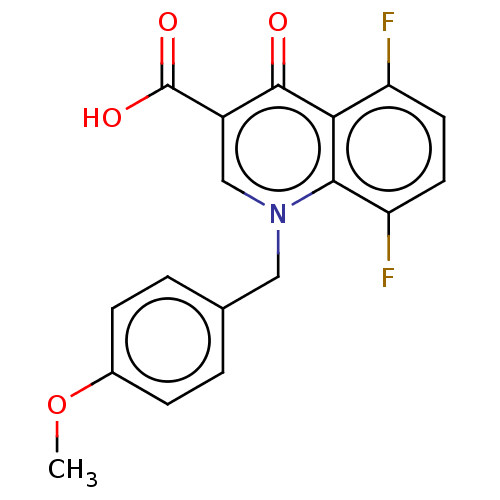 (CHEMBL594432) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 acetylcholine receptor expressed CHO cells assessed as intracellular calcium mobilization after 1 hr by Fluo-... | J Med Chem 56: 5151-72 (2013) Article DOI: 10.1021/jm400540b BindingDB Entry DOI: 10.7270/Q2WS8X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50062570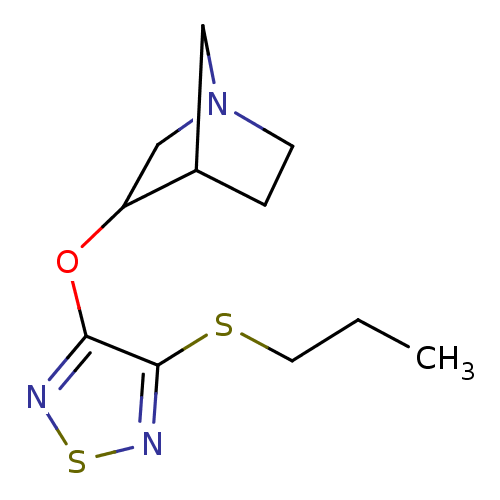 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Effective concentration required for stimulation of phosphoinositol (PI) hydrolysis in the A9 L cell line transfected with the Muscarinic acetylcholi... | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50062570 (3-(4-Propylsulfanyl-[1,2,5]thiadiazol-3-yloxy)-1-a...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Effective concentration required for stimulation of phosphoinositol (PI) hydrolysis in the A9 L cell line transfected with the Muscarinic acetylcholi... | J Med Chem 41: 379-92 (1998) Article DOI: 10.1021/jm970125n BindingDB Entry DOI: 10.7270/Q2SX6CBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM261732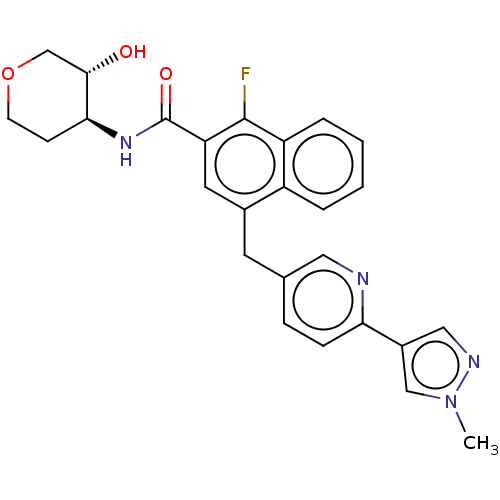 (1-fluoro-N- ((3RS,4SR)-3- hydroxytetrahydro- 2H-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
HOFFMANN-LA-ROCHE INC. US Patent | Assay Description The assay is designed to select compounds that possess modulator activity at the acetylcholine muscarinic receptor expressed in CHO cells by measurin... | US Patent US9708302 (2017) BindingDB Entry DOI: 10.7270/Q2BV7JMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Partial agonist activity at human muscarinic M1 acetylcholine receptor expressed in CHO cells assessed as increase in IP1 accumulation incubated for ... | J Med Chem 58: 560-76 (2015) Article DOI: 10.1021/jm500860w BindingDB Entry DOI: 10.7270/Q2125VCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50263888 (CHEMBL491209 | Ethyl 4-(2-oxo-2,3-dihydro-1Hbenzo[...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50077547 (1-{4-[3-(3-Fluoro-phenyl)-prop-2-ynylsulfanyl]-[1,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Stimulation of phosphoinositol hydrolysis in the mouse fibroblast cell line A9L-M1 expressing Muscarinic acetylcholine receptor M1. | J Med Chem 42: 1999-2006 (1999) Article DOI: 10.1021/jm9910019 BindingDB Entry DOI: 10.7270/Q2XD12BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416730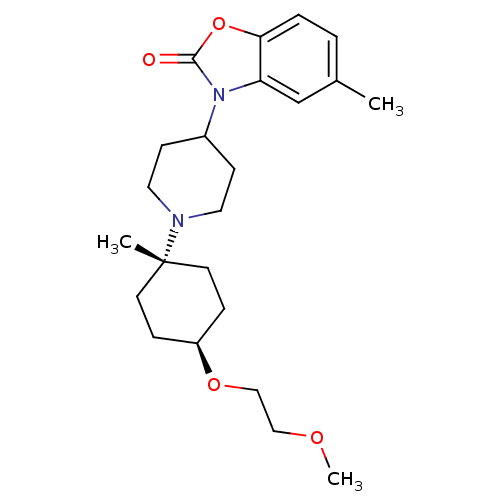 (CHEMBL1223940) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416729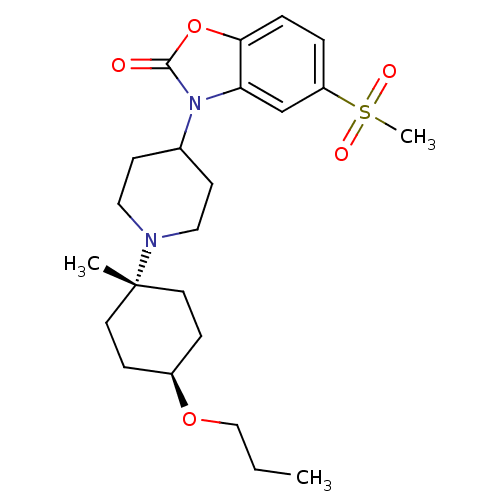 (CHEMBL1223939) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416725 (CHEMBL1223862) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM261732 (1-fluoro-N- ((3RS,4SR)-3- hydroxytetrahydro- 2H-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
HOFFMANN-LA-ROCHE INC. US Patent | Assay Description The assay is designed to select compounds that possess modulator activity at the acetylcholine muscarinic receptor expressed in CHO cells by measurin... | US Patent US9708302 (2017) BindingDB Entry DOI: 10.7270/Q2BV7JMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416726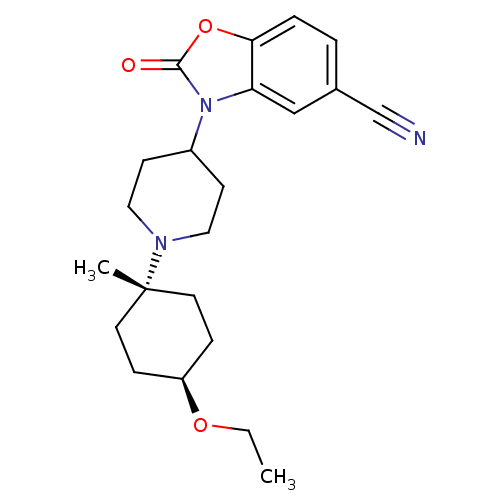 (CHEMBL1223863) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416735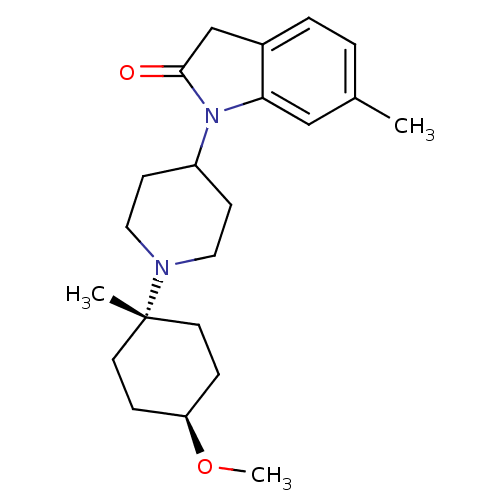 (CHEMBL1223805) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50416140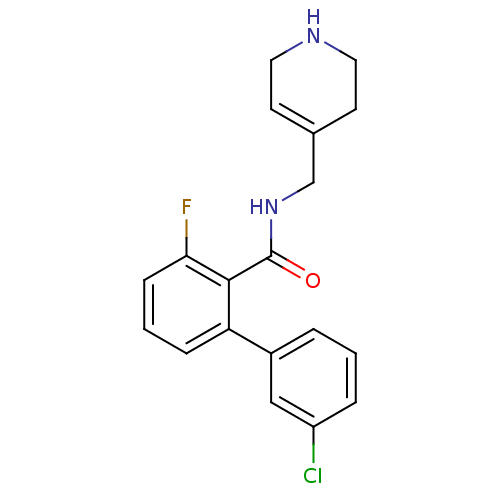 (CHEMBL1083618) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M1 receptor expressed in CHO cells assessed as effect on acetylcholine-induced intracellular calcium level by FL... | Bioorg Med Chem Lett 20: 3545-9 (2010) Article DOI: 10.1016/j.bmcl.2010.04.127 BindingDB Entry DOI: 10.7270/Q2QF8V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193784 (US9670209, Compound 302 1-((R)-3-((1R,3R,5S)-3-(cy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | 25 |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description Compounds were loaded in a 384 Falcon v-bottom plate (0.5 ul/well in a 11 point, 3-fold dilution). Membranes prepared from S1P1/CHO cells or EDG3-Gal... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM193785 (US9670209, Compound 303 1-((R)-3-((1R,3R,5S)-3-(2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1258 total ) | Next | Last >> |