 Found 108 hits of ec50 data for polymerid = 1622
Found 108 hits of ec50 data for polymerid = 1622 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336882

(CHEMBL1672327 | N6-(6-(1-methylpiperidin-4-yloxy)p...)Show SMILES CN1CCC(CC1)Oc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cnc(N)nc2)cn1 Show InChI InChI=1S/C23H29N9O2/c1-31-6-4-18(5-7-31)34-21-3-2-17(15-25-21)28-20-12-19(16-13-26-22(24)27-14-16)29-23(30-20)32-8-10-33-11-9-32/h2-3,12-15,18H,4-11H2,1H3,(H2,24,26,27)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50426176
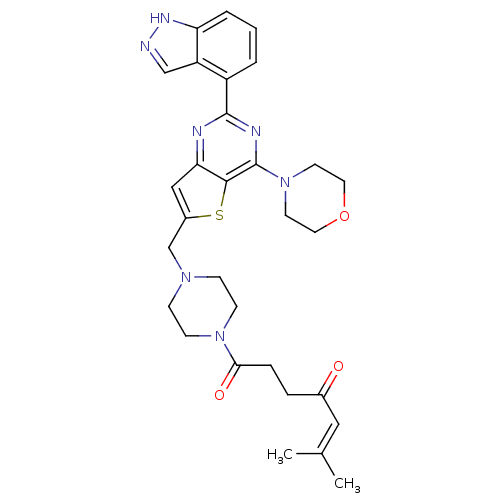
(CHEMBL2316958)Show SMILES CC(C)=CC(=O)CCC(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 |(2.18,-16.72,;2.94,-15.38,;2.16,-14.06,;4.48,-15.37,;5.24,-14.03,;4.46,-12.71,;6.78,-14.02,;7.54,-12.68,;9.08,-12.67,;9.86,-14,;9.84,-11.33,;11.38,-11.32,;12.14,-9.98,;11.37,-8.66,;12.13,-7.32,;13.67,-7.31,;14.58,-8.56,;16.04,-8.08,;17.38,-8.84,;18.71,-8.06,;18.69,-6.52,;17.36,-5.76,;17.35,-4.23,;18.68,-3.45,;18.67,-1.92,;17.33,-1.15,;16,-1.93,;16.01,-3.47,;16.04,-6.54,;14.57,-6.07,;20.04,-8.82,;20.05,-10.36,;21.39,-11.12,;22.72,-10.34,;22.7,-8.79,;23.82,-7.76,;23.19,-6.37,;21.67,-6.54,;21.36,-8.04,;9.83,-8.66,;9.07,-10,)| Show InChI InChI=1S/C30H35N7O3S/c1-20(2)16-21(38)6-7-27(39)36-10-8-35(9-11-36)19-22-17-26-28(41-22)30(37-12-14-40-15-13-37)33-29(32-26)23-4-3-5-25-24(23)18-31-34-25/h3-5,16-18H,6-15,19H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Celgene Avilomics Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human SKOV3 cells assessed as inhibition of Akt Ser473 phosphorylation at 1 to 1000 nM after 1 hr by immunoblot analysis |
J Med Chem 56: 712-21 (2013)
Article DOI: 10.1021/jm3008745
BindingDB Entry DOI: 10.7270/Q2MG7QVK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
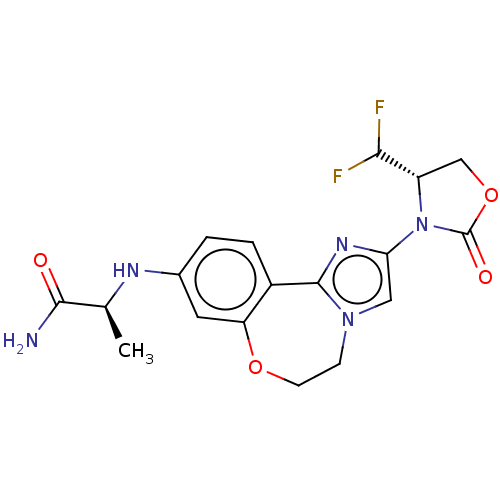
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295669
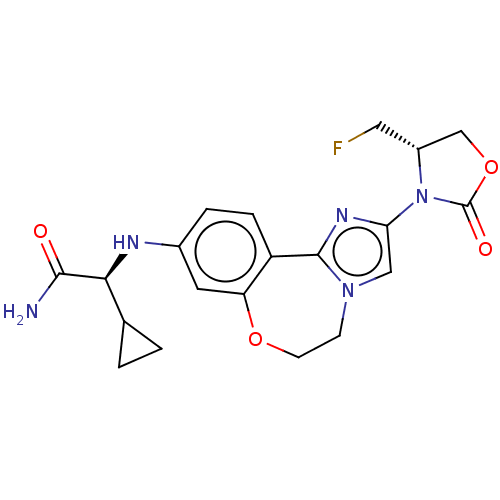
((S)-2-cyclopropyl-2-((2-((S)-4- (fluoromethyl)-2-o...)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O)C1CC1 |r| Show InChI InChI=1S/C20H22FN5O4/c21-8-13-10-30-20(28)26(13)16-9-25-5-6-29-15-7-12(3-4-14(15)19(25)24-16)23-17(18(22)27)11-1-2-11/h3-4,7,9,11,13,17,23H,1-2,5-6,8,10H2,(H2,22,27)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM475607
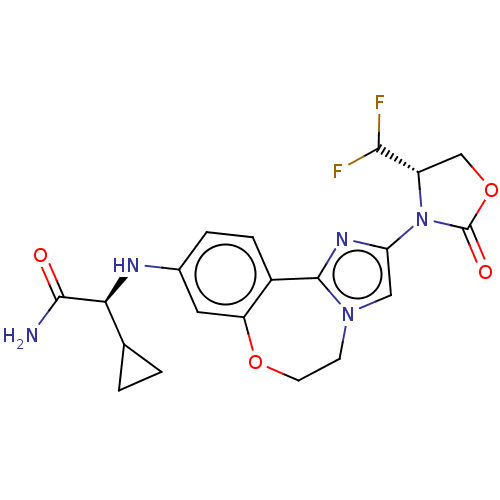
(US10851091, Compound 103)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C1CC1 |r| Show InChI InChI=1S/C20H21F2N5O4/c21-17(22)13-9-31-20(29)27(13)15-8-26-5-6-30-14-7-11(3-4-12(14)19(26)25-15)24-16(18(23)28)10-1-2-10/h3-4,7-8,10,13,16-17,24H,1-2,5-6,9H2,(H2,23,28)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602305
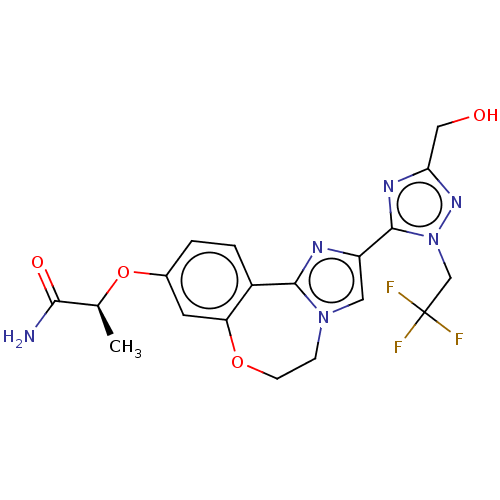
(CHEMBL5209048)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1nc(CO)nn1CC(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
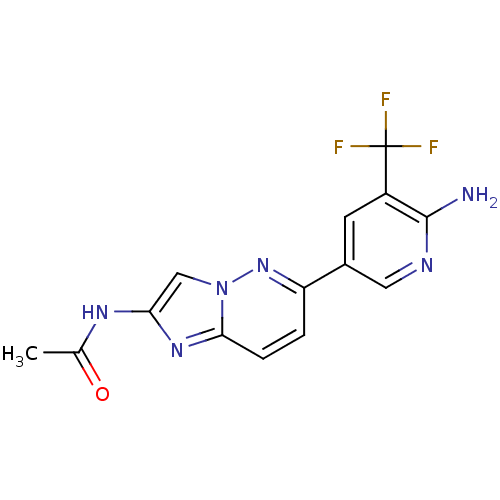
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140272
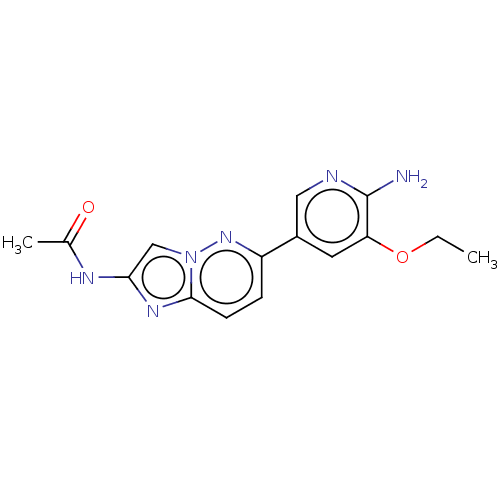
(CHEMBL3754572)Show InChI InChI=1S/C15H16N6O2/c1-3-23-12-6-10(7-17-15(12)16)11-4-5-14-19-13(18-9(2)22)8-21(14)20-11/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140269
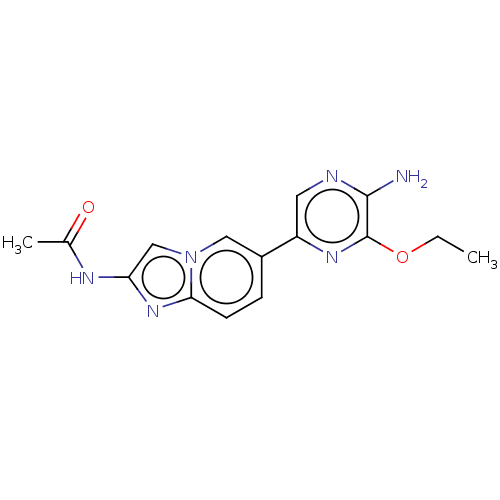
(CHEMBL3752653)Show InChI InChI=1S/C15H16N6O2/c1-3-23-15-14(16)17-6-11(19-15)10-4-5-13-20-12(18-9(2)22)8-21(13)7-10/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140267
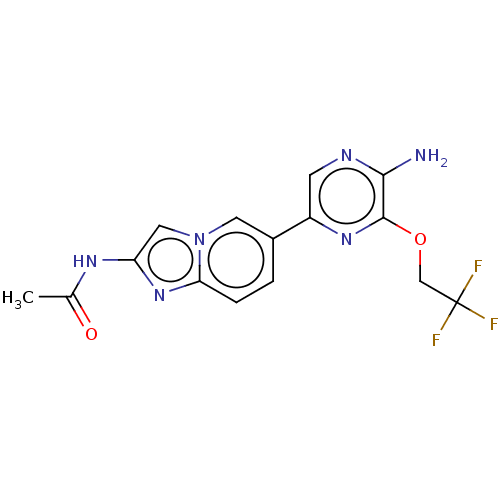
(CHEMBL3752019)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C15H13F3N6O2/c1-8(25)21-11-6-24-5-9(2-3-12(24)23-11)10-4-20-13(19)14(22-10)26-7-15(16,17)18/h2-6H,7H2,1H3,(H2,19,20)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
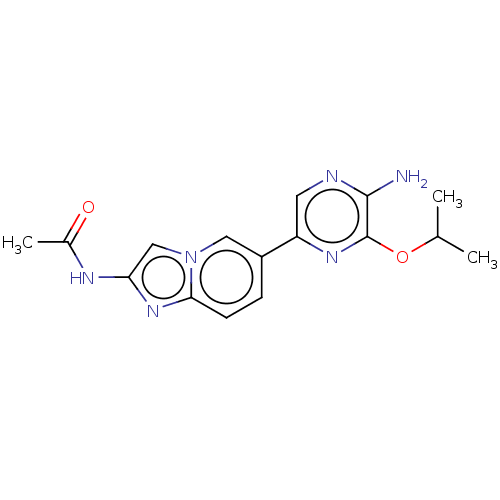
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140265
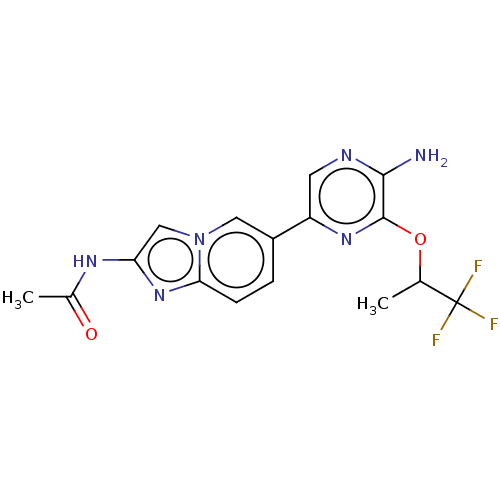
(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | <40 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602321
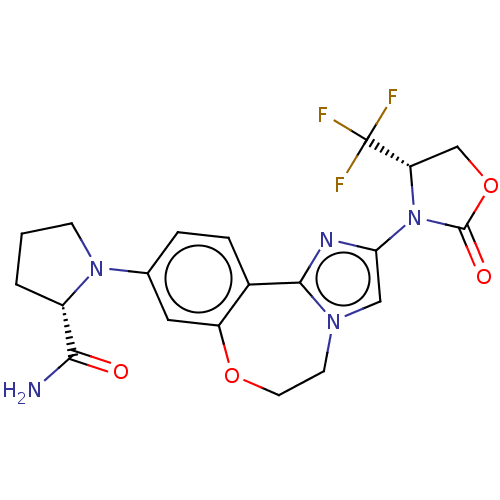
(CHEMBL5202305)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of wild type PI3Kalpha (unknown origin) after 40 mins by kinase-Glo reagent based luminescence assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336880

(2-morpholino-N6-(quinolin-3-yl)-4,5'-bipyrimidine-...)Show SMILES Nc1ncc(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H20N8O/c22-20-24-11-15(12-25-20)18-10-19(28-21(27-18)29-5-7-30-8-6-29)26-16-9-14-3-1-2-4-17(14)23-13-16/h1-4,9-13H,5-8H2,(H2,22,24,25)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602328
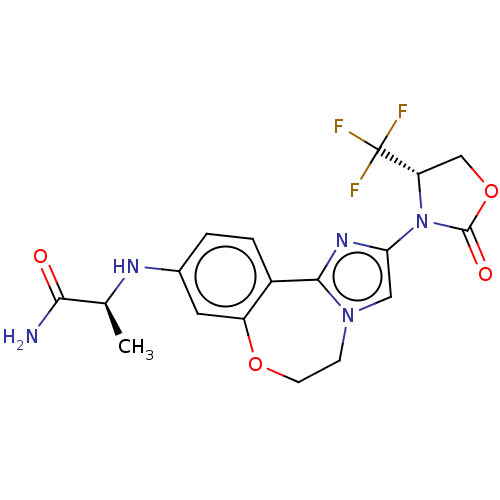
(CHEMBL5205438)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315213
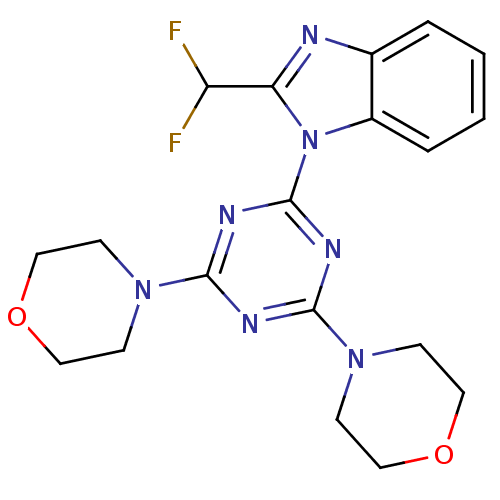
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of wild type PI3Kalpha (unknown origin) after 40 mins by kinase-Glo reagent based luminescence assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha H1047R mutant (unknown origin) by HTRF assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602331
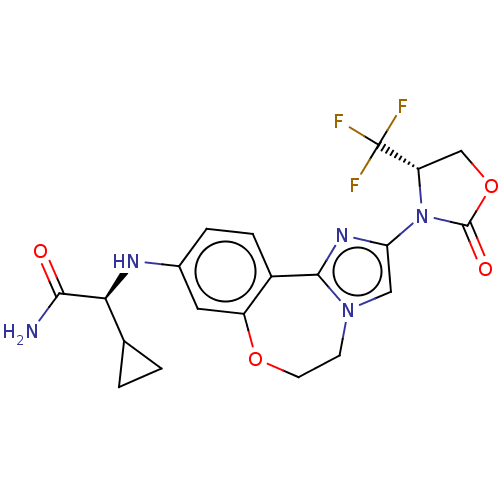
(CHEMBL5182371)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602329
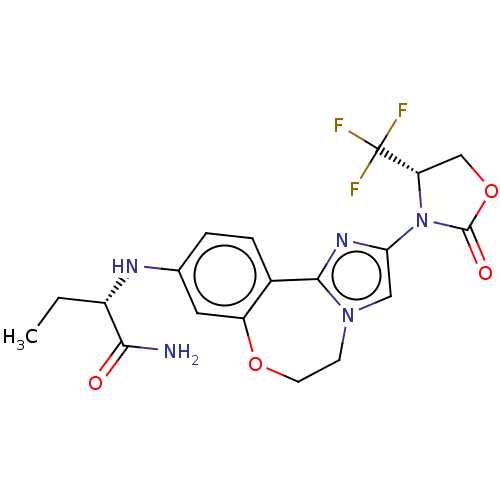
(CHEMBL5189517)Show SMILES CC[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140270
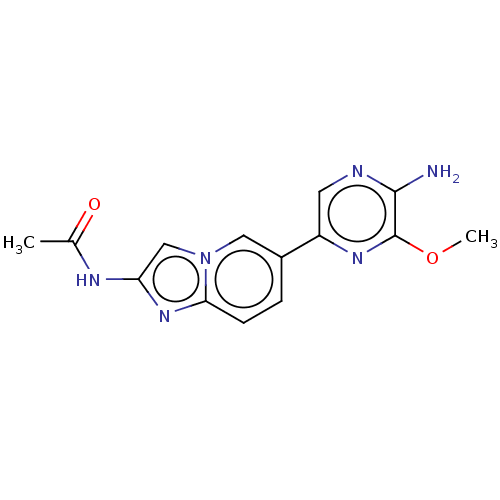
(CHEMBL3752503)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-11-7-20-6-9(3-4-12(20)19-11)10-5-16-13(15)14(18-10)22-2/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602308
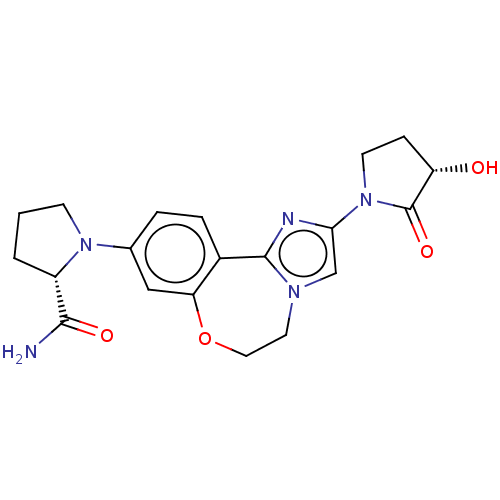
(CHEMBL5192172)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602304
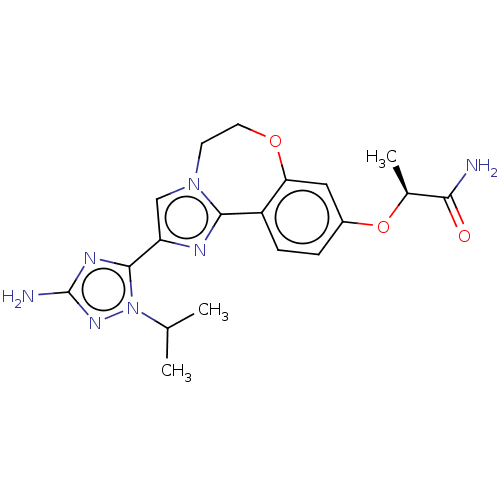
(CHEMBL5182339)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711
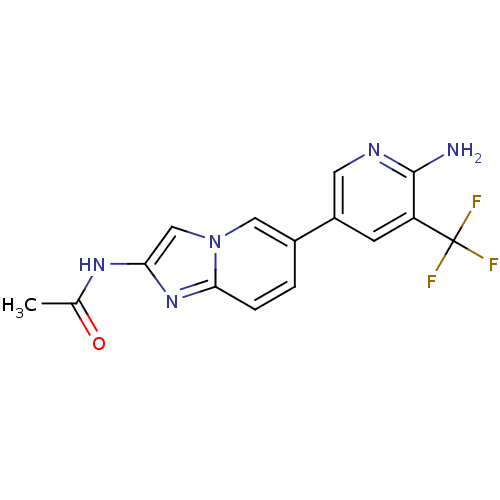
(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149548
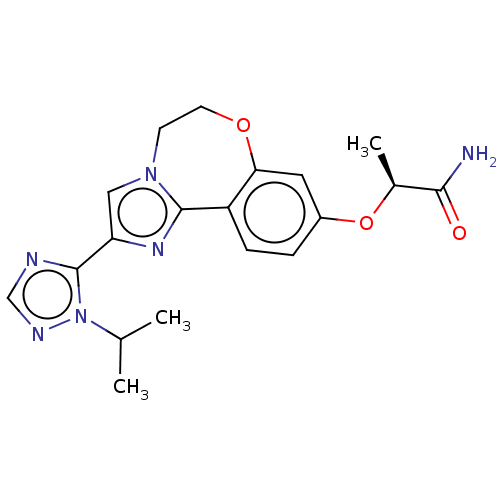
(CHEMBL3771364 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(O[C@@H](C)C(N)=O)ccc3-c2n1 |r| Show InChI InChI=1S/C19H22N6O3/c1-11(2)25-19(21-10-22-25)15-9-24-6-7-27-16-8-13(28-12(3)17(20)26)4-5-14(16)18(24)23-15/h4-5,8-12H,6-7H2,1-3H3,(H2,20,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 89 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336865
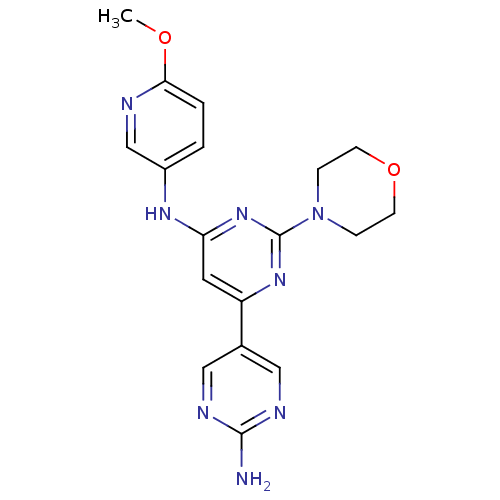
(CHEMBL1672328 | N6-(6-methoxypyridin-3-yl)-2-morph...)Show SMILES COc1ccc(Nc2cc(nc(n2)N2CCOCC2)-c2cnc(N)nc2)cn1 Show InChI InChI=1S/C18H20N8O2/c1-27-16-3-2-13(11-20-16)23-15-8-14(12-9-21-17(19)22-10-12)24-18(25-15)26-4-6-28-7-5-26/h2-3,8-11H,4-7H2,1H3,(H2,19,21,22)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140275

(CHEMBL3752775)Show InChI InChI=1S/C15H15N5O2/c1-9(21)18-13-8-20-7-10(3-4-14(20)19-13)11-5-12(22-2)15(16)17-6-11/h3-8H,1-2H3,(H2,16,17)(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140273
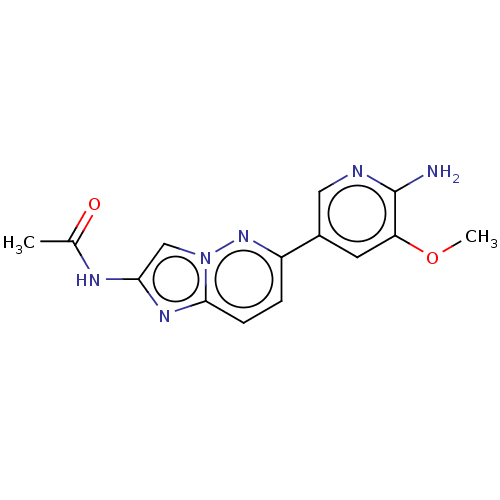
(CHEMBL3752760)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-12-7-20-13(18-12)4-3-10(19-20)9-5-11(22-2)14(15)16-6-9/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336875
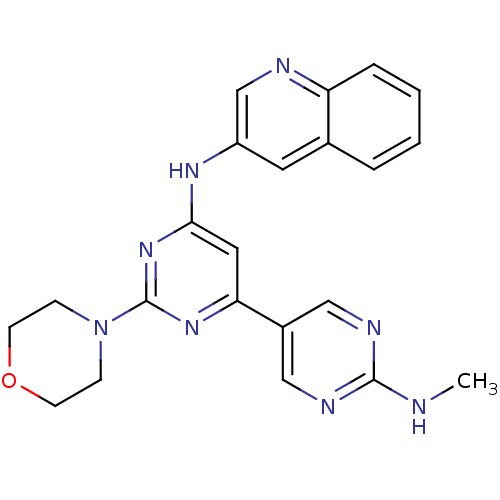
(CHEMBL1672324 | N2'-methyl-2-morpholino-N6-(quinol...)Show SMILES CNc1ncc(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H22N8O/c1-23-21-25-12-16(13-26-21)19-11-20(29-22(28-19)30-6-8-31-9-7-30)27-17-10-15-4-2-3-5-18(15)24-14-17/h2-5,10-14H,6-9H2,1H3,(H,23,25,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336881

(2-morpholino-N6-(6-phenoxypyridin-3-yl)-4,5'-bipyr...)Show SMILES Nc1ncc(cn1)-c1cc(Nc2ccc(Oc3ccccc3)nc2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H22N8O2/c24-22-26-13-16(14-27-22)19-12-20(30-23(29-19)31-8-10-32-11-9-31)28-17-6-7-21(25-15-17)33-18-4-2-1-3-5-18/h1-7,12-15H,8-11H2,(H2,24,26,27)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336879
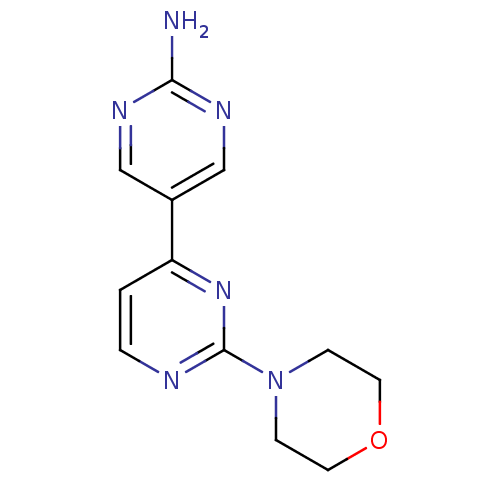
(2-morpholino-4,5'-bipyrimidin-2'-amine | CHEMBL167...)Show InChI InChI=1S/C12H14N6O/c13-11-15-7-9(8-16-11)10-1-2-14-12(17-10)18-3-5-19-6-4-18/h1-2,7-8H,3-6H2,(H2,13,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140271
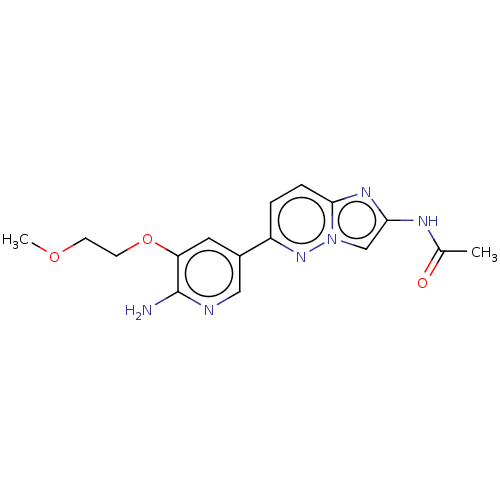
(CHEMBL3751961)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-14-9-22-15(20-14)4-3-12(21-22)11-7-13(16(17)18-8-11)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602330
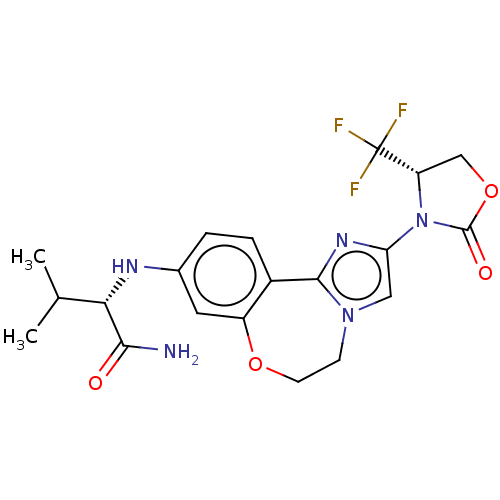
(CHEMBL5186223)Show SMILES CC(C)[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 157 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140268

(CHEMBL3753085)Show InChI InChI=1S/C16H18N6O3/c1-10(23)19-13-9-22-8-11(3-4-14(22)21-13)12-7-18-15(17)16(20-12)25-6-5-24-2/h3-4,7-9H,5-6H2,1-2H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341209
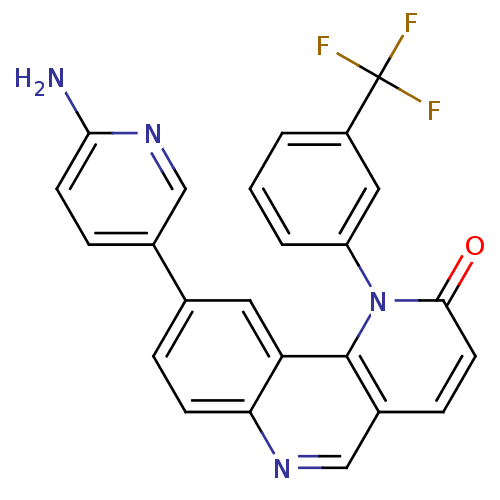
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336878
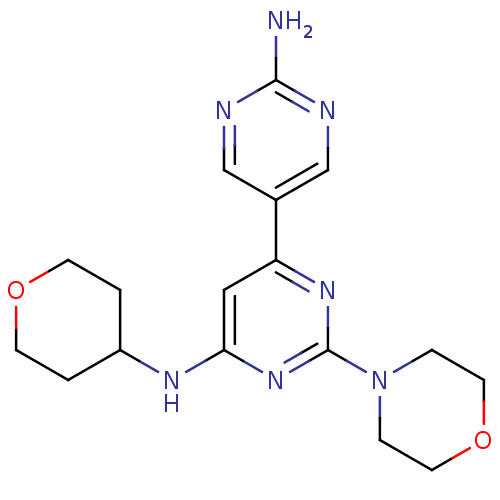
(2-morpholino-N6-(tetrahydro-2H-pyran-4-yl)-4,5'-bi...)Show InChI InChI=1S/C17H23N7O2/c18-16-19-10-12(11-20-16)14-9-15(21-13-1-5-25-6-2-13)23-17(22-14)24-3-7-26-8-4-24/h9-11,13H,1-8H2,(H2,18,19,20)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341215
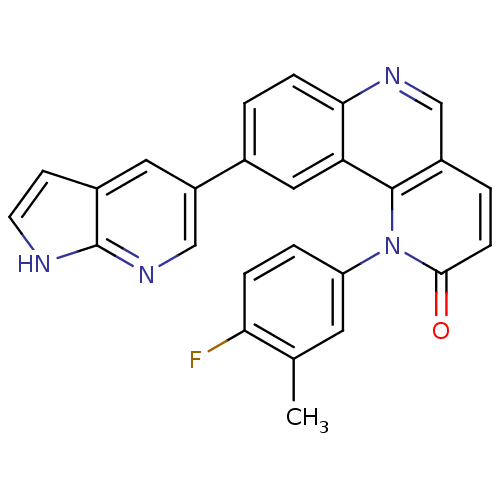
(1-(4-fluoro-3-methylphenyl)-9-(1H-pyrrolo[2,3-b]py...)Show SMILES Cc1cc(ccc1F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C26H17FN4O/c1-15-10-20(4-5-22(15)27)31-24(32)7-3-18-13-29-23-6-2-16(12-21(23)25(18)31)19-11-17-8-9-28-26(17)30-14-19/h2-14H,1H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341216

(9-(6-aminopyridin-3-yl)-1-(3-chloro-4-fluorophenyl...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4ccc(F)c(Cl)c4)c3c2c1 Show InChI InChI=1S/C23H14ClFN4O/c24-18-10-16(4-5-19(18)25)29-22(30)8-3-15-12-27-20-6-1-13(9-17(20)23(15)29)14-2-7-21(26)28-11-14/h1-12H,(H2,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341210
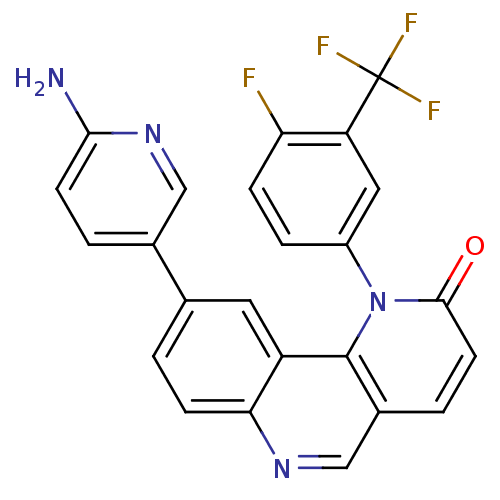
(9-(6-aminopyridin-3-yl)-1-(4-fluoro-3-(trifluorome...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4ccc(F)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H14F4N4O/c25-19-5-4-16(10-18(19)24(26,27)28)32-22(33)8-3-15-12-30-20-6-1-13(9-17(20)23(15)32)14-2-7-21(29)31-11-14/h1-12H,(H2,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140274

(CHEMBL3753665)Show InChI InChI=1S/C16H17N5O2/c1-3-23-13-6-12(7-18-16(13)17)11-4-5-15-20-14(19-10(2)22)9-21(15)8-11/h4-9H,3H2,1-2H3,(H2,17,18)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human A2780 cells assessed as reduction of AKT phosphorylation at Ser473 after 1 hr |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336868
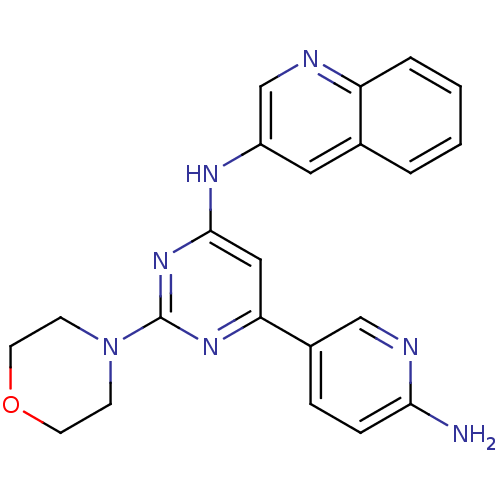
(CHEMBL1672316 | N-(6-(6-aminopyridin-3-yl)-2-morph...)Show SMILES Nc1ccc(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H21N7O/c23-20-6-5-16(13-25-20)19-12-21(28-22(27-19)29-7-9-30-10-8-29)26-17-11-15-3-1-2-4-18(15)24-14-17/h1-6,11-14H,7-10H2,(H2,23,25)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602308

(CHEMBL5192172)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1CC[C@H](O)C1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 327 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336866
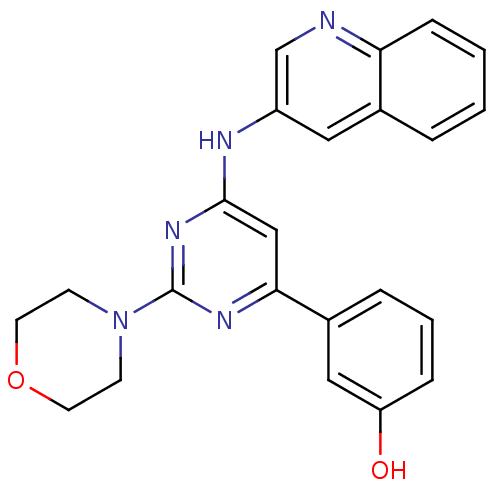
(3-(2-morpholino-6-(quinolin-3-ylamino)pyrimidin-4-...)Show SMILES Oc1cccc(c1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H21N5O2/c29-19-6-3-5-17(13-19)21-14-22(27-23(26-21)28-8-10-30-11-9-28)25-18-12-16-4-1-2-7-20(16)24-15-18/h1-7,12-15,29H,8-11H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336871

(CHEMBL1672319 | N-(2-morpholino-6-(pyrazin-2-yl)py...)Show SMILES C1CN(CCO1)c1nc(Nc2cnc3ccccc3c2)cc(n1)-c1cnccn1 Show InChI InChI=1S/C21H19N7O/c1-2-4-17-15(3-1)11-16(13-24-17)25-20-12-18(19-14-22-5-6-23-19)26-21(27-20)28-7-9-29-10-8-28/h1-6,11-14H,7-10H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50336873
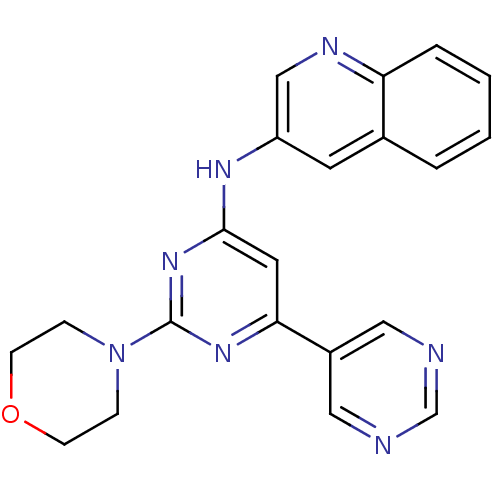
(CHEMBL1672321 | N-(2-morpholino-4,5'-bipyrimidin-6...)Show SMILES C1CN(CCO1)c1nc(Nc2cnc3ccccc3c2)cc(n1)-c1cncnc1 Show InChI InChI=1S/C21H19N7O/c1-2-4-18-15(3-1)9-17(13-24-18)25-20-10-19(16-11-22-14-23-12-16)26-21(27-20)28-5-7-29-8-6-28/h1-4,9-14H,5-8H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha-mediated Akt phosphorylation at Ser473 in human A2780 cells after 1 hr |
ACS Med Chem Lett 2: 34-38 (2011)
Article DOI: 10.1021/ml1001932
BindingDB Entry DOI: 10.7270/Q2B56K0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341211

(9-(2-aminopyrimidin-5-yl)-1-(4-fluoro-3-(trifluoro...)Show SMILES Nc1ncc(cn1)-c1ccc2ncc3ccc(=O)n(-c4ccc(F)c(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C23H13F4N5O/c24-18-4-3-15(8-17(18)23(25,26)27)32-20(33)6-2-13-9-29-19-5-1-12(7-16(19)21(13)32)14-10-30-22(28)31-11-14/h1-11H,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341222
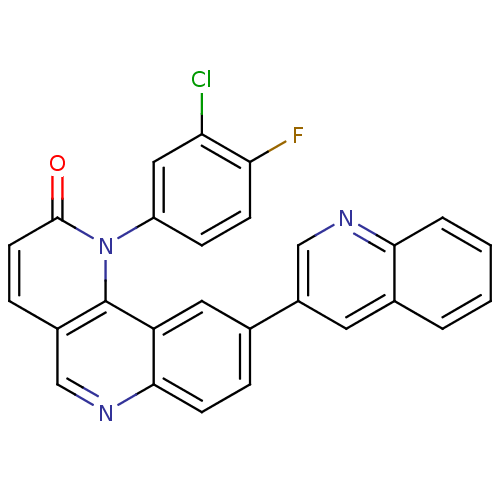
(1-(3-chloro-4-fluorophenyl)-9-(quinolin-3-yl)benzo...)Show SMILES Fc1ccc(cc1Cl)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 Show InChI InChI=1S/C27H15ClFN3O/c28-22-13-20(7-8-23(22)29)32-26(33)10-6-18-14-31-25-9-5-16(12-21(25)27(18)32)19-11-17-3-1-2-4-24(17)30-15-19/h1-15H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341226

(1-(4-fluoro-3-(trifluoromethyl)phenyl)-9-(1H-indaz...)Show SMILES Fc1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C26H14F4N4O/c27-21-5-4-18(11-20(21)26(28,29)30)34-24(35)8-3-16-12-31-23-7-2-15(10-19(23)25(16)34)14-1-6-22-17(9-14)13-32-33-22/h1-13H,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha in human PC3 cells expressing Akt1 S473D mutant assessed as phosphorylation of Akt Thr308 by immunoblotting |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50467448

(CHEMBL4280001)Show SMILES Oc1ccc(-c2n[nH]cc2-c2ccc(Cl)c(Cl)c2)c(O)c1O Show InChI InChI=1S/C15H10Cl2N2O3/c16-10-3-1-7(5-11(10)17)9-6-18-19-13(9)8-2-4-12(20)15(22)14(8)21/h1-6,20-22H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha H1047R mutant (unknown origin) by HTRF assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data