 Found 247 hits of ec50 data for polymerid = 1952
Found 247 hits of ec50 data for polymerid = 1952 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18887
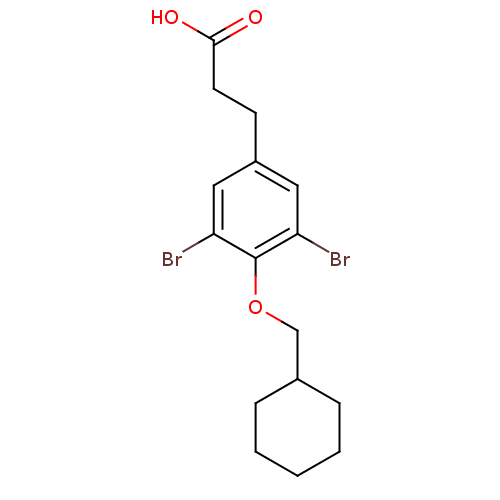
(3,5-Dibromo-4-alkoxyphenylalkanoic Acid, 9k | 3-[3...)Show InChI InChI=1S/C16H20Br2O3/c17-13-8-12(6-7-15(19)20)9-14(18)16(13)21-10-11-4-2-1-3-5-11/h8-9,11H,1-7,10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | 0.120 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 48: 3114-7 (2005)
Article DOI: 10.1021/jm050004k
BindingDB Entry DOI: 10.7270/Q2M906XW |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867
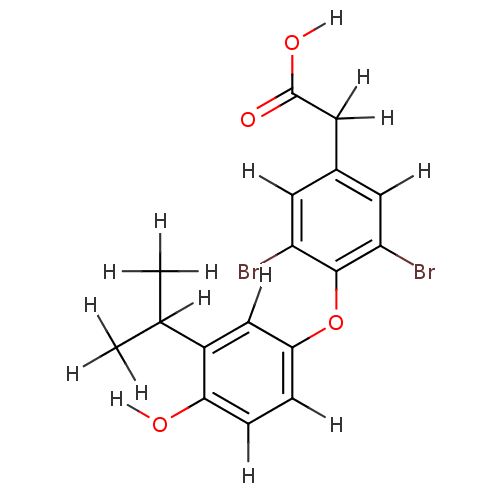
(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | 0.200 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18867

(2-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...)Show InChI InChI=1S/C17H16Br2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | 0.200 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18870
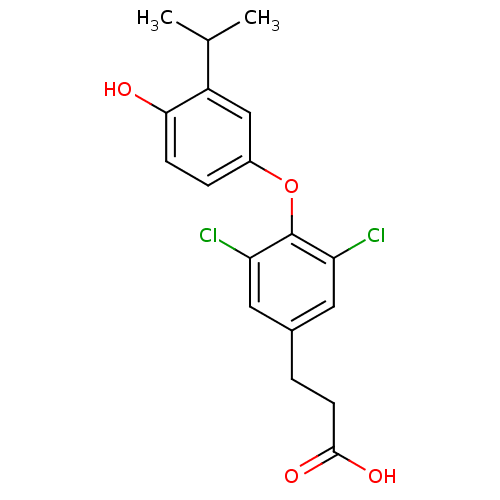
(3-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-9-12(4-5-16(13)21)24-18-14(19)7-11(8-15(18)20)3-6-17(22)23/h4-5,7-10,21H,3,6H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.150 | n/a | 0.280 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50401075
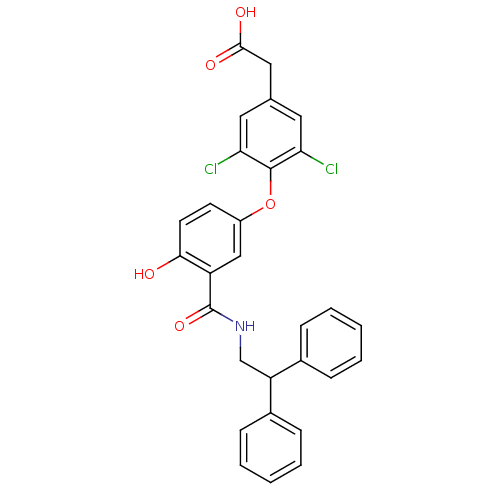
(CHEMBL2203702)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)C(=O)NCC(c2ccccc2)c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C29H23Cl2NO5/c30-24-13-18(15-27(34)35)14-25(31)28(24)37-21-11-12-26(33)22(16-21)29(36)32-17-23(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-14,16,23,33H,15,17H2,(H,32,36)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18904
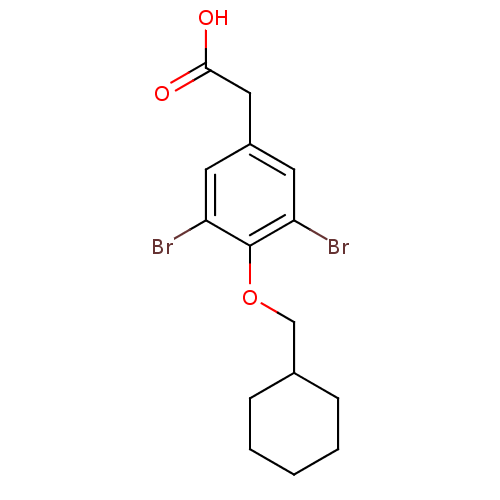
(2-[3,5-dibromo-4-(cyclohexylmethoxy)phenyl]acetic ...)Show InChI InChI=1S/C15H18Br2O3/c16-12-6-11(8-14(18)19)7-13(17)15(12)20-9-10-4-2-1-3-5-10/h6-7,10H,1-5,8-9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | 0.520 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 48: 3114-7 (2005)
Article DOI: 10.1021/jm050004k
BindingDB Entry DOI: 10.7270/Q2M906XW |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18903

(2-[3,5-dibromo-4-(2-ethylbutoxy)phenyl]acetic acid...)Show InChI InChI=1S/C14H18Br2O3/c1-3-9(4-2)8-19-14-11(15)5-10(6-12(14)16)7-13(17)18/h5-6,9H,3-4,7-8H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | 0.700 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 48: 3114-7 (2005)
Article DOI: 10.1021/jm050004k
BindingDB Entry DOI: 10.7270/Q2M906XW |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18877
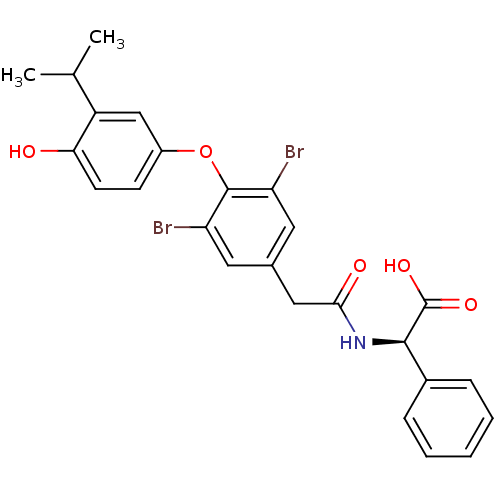
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@@H](C(O)=O)c3ccccc3)cc2Br)ccc1O |r| Show InChI InChI=1S/C25H23Br2NO5/c1-14(2)18-13-17(8-9-21(18)29)33-24-19(26)10-15(11-20(24)27)12-22(30)28-23(25(31)32)16-6-4-3-5-7-16/h3-11,13-14,23,29H,12H2,1-2H3,(H,28,30)(H,31,32)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | 0.730 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18874

((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | 0.770 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
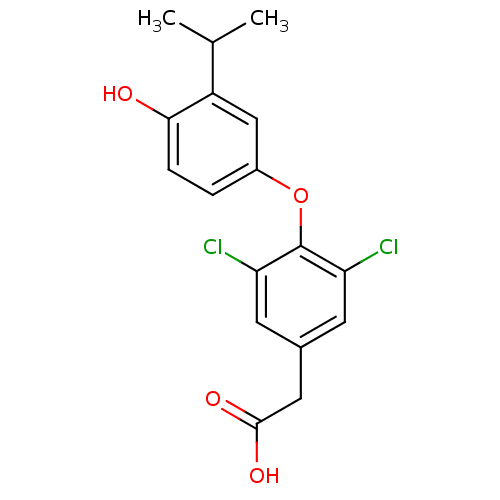
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | 0.780 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18902
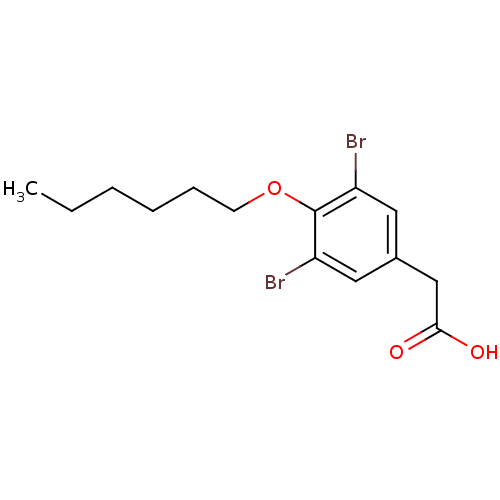
(2-[3,5-dibromo-4-(hexyloxy)phenyl]acetic acid | 3,...)Show InChI InChI=1S/C14H18Br2O3/c1-2-3-4-5-6-19-14-11(15)7-10(8-12(14)16)9-13(17)18/h7-8H,2-6,9H2,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | 0.800 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 48: 3114-7 (2005)
Article DOI: 10.1021/jm050004k
BindingDB Entry DOI: 10.7270/Q2M906XW |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM426634
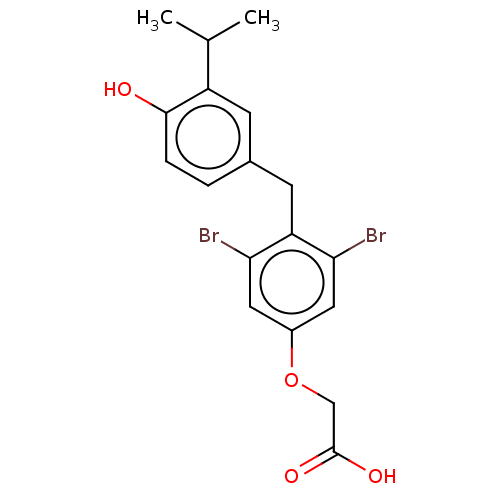
(US10544075, Compound JD-20)Show InChI InChI=1S/C18H18Br2O4/c1-10(2)13-5-11(3-4-17(13)21)6-14-15(19)7-12(8-16(14)20)24-9-18(22)23/h3-5,7-8,10,21H,6,9H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a |
OREGON HEALTH & SCIENCE UNIVERSITY
US Patent
| Assay Description
Human epithelial kidney cells (HEK 293) were grown to 80% confluency in Dubelcco's modified Eagles 4.5 g/L glucose medium (high glucose DMEM) con... |
US Patent US10544075 (2020)
BindingDB Entry DOI: 10.7270/Q2NP26SZ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18906
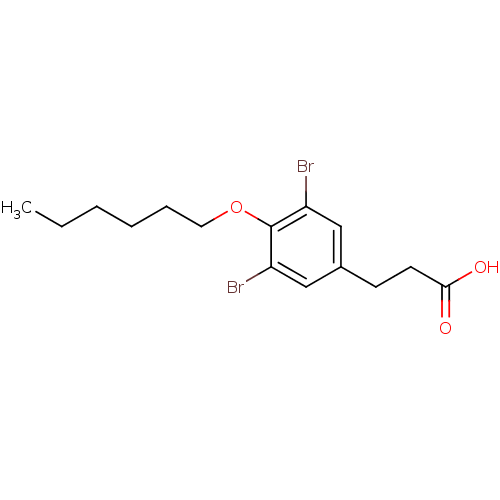
(3,5-Dibromo-4-alkoxyphenylalkanoic Acid, 9i | 3-[3...)Show InChI InChI=1S/C15H20Br2O3/c1-2-3-4-5-8-20-15-12(16)9-11(10-13(15)17)6-7-14(18)19/h9-10H,2-8H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | 1 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 48: 3114-7 (2005)
Article DOI: 10.1021/jm050004k
BindingDB Entry DOI: 10.7270/Q2M906XW |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Agonist activity at human thyroid hormone receptor beta expressed in CV1 cells by TRE-luciferase assay |
Bioorg Med Chem Lett 18: 3919-24 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.038
BindingDB Entry DOI: 10.7270/Q2CZ36ZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18875
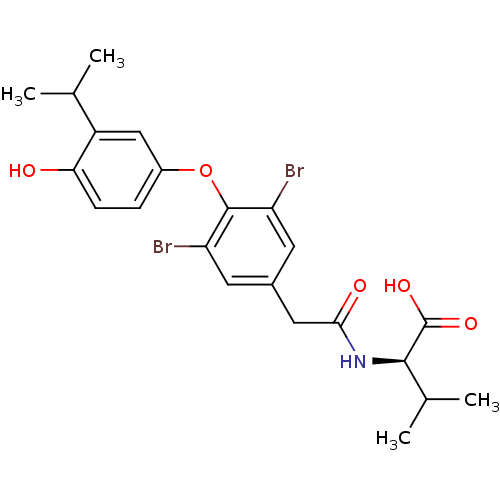
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)[C@@H](NC(=O)Cc1cc(Br)c(Oc2ccc(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H25Br2NO5/c1-11(2)15-10-14(5-6-18(15)26)30-21-16(23)7-13(8-17(21)24)9-19(27)25-20(12(3)4)22(28)29/h5-8,10-12,20,26H,9H2,1-4H3,(H,25,27)(H,28,29)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | 1.20 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM426635
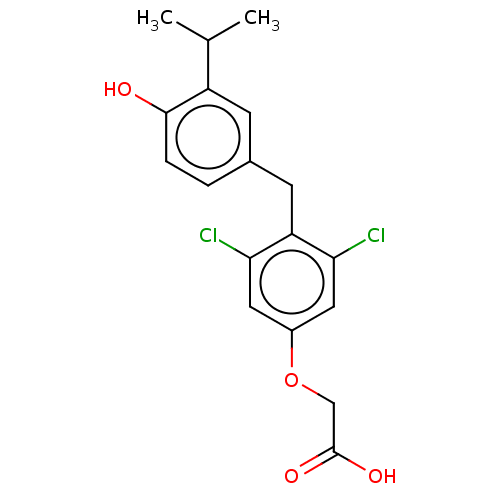
(US10544075, Compound JD-21)Show InChI InChI=1S/C18H18Cl2O4/c1-10(2)13-5-11(3-4-17(13)21)6-14-15(19)7-12(8-16(14)20)24-9-18(22)23/h3-5,7-8,10,21H,6,9H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a |
OREGON HEALTH & SCIENCE UNIVERSITY
US Patent
| Assay Description
Human epithelial kidney cells (HEK 293) were grown to 80% confluency in Dubelcco's modified Eagles 4.5 g/L glucose medium (high glucose DMEM) con... |
US Patent US10544075 (2020)
BindingDB Entry DOI: 10.7270/Q2NP26SZ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a |
OREGON HEALTH & SCIENCE UNIVERSITY
US Patent
| Assay Description
Human epithelial kidney cells (HEK 293) were grown to 80% confluency in Dubelcco's modified Eagles 4.5 g/L glucose medium (high glucose DMEM) con... |
US Patent US10544075 (2020)
BindingDB Entry DOI: 10.7270/Q2NP26SZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration of the compound binding towards TRbeta1 in E25B2 cells (agonistic activity) |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Sanwa Kagaku Kenkyusho Co., Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant TRbeta1 transfected in CV-1 cells after 8 to 10 hrs by alkaline phosphatase reporter gene assay |
Bioorg Med Chem 22: 488-98 (2013)
Article DOI: 10.1016/j.bmc.2013.11.001
BindingDB Entry DOI: 10.7270/Q2H41SXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Half-maximum activation of human Thyroid hormone receptor beta 1 (hTRbeta1) |
J Med Chem 45: 3310-20 (2002)
BindingDB Entry DOI: 10.7270/Q20G3JHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Effect on human TRbeta transactivation activity in HeLa cells by luciferase reporter assay |
Bioorg Med Chem 16: 762-70 (2008)
Article DOI: 10.1016/j.bmc.2007.10.040
BindingDB Entry DOI: 10.7270/Q2RR2034 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of California-San Francisco
| Assay Description
TRE-driven dual-luciferase reporter assay in human uterine cervical cancer (Hela) cell line |
ACS Chem Biol 1: 585-93 (2006)
Article DOI: 10.1021/cb600311v
BindingDB Entry DOI: 10.7270/Q23N21R4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605643
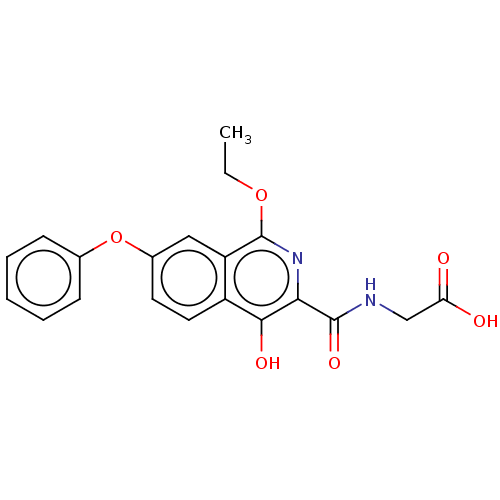
(CHEMBL5172845)Show SMILES CCOc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18872
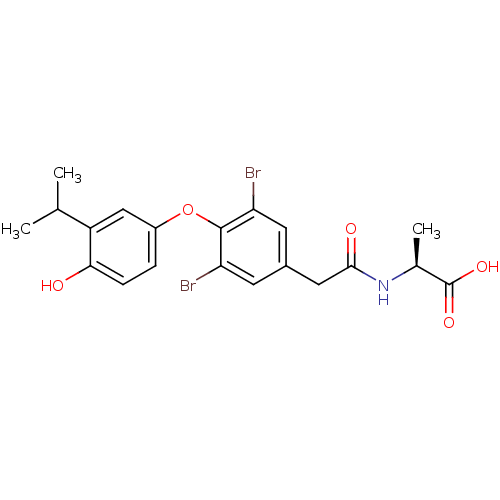
((2S)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@@H](C)C(O)=O)cc2Br)ccc1O |r| Show InChI InChI=1S/C20H21Br2NO5/c1-10(2)14-9-13(4-5-17(14)24)28-19-15(21)6-12(7-16(19)22)8-18(25)23-11(3)20(26)27/h4-7,9-11,24H,8H2,1-3H3,(H,23,25)(H,26,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | 2.60 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50115668
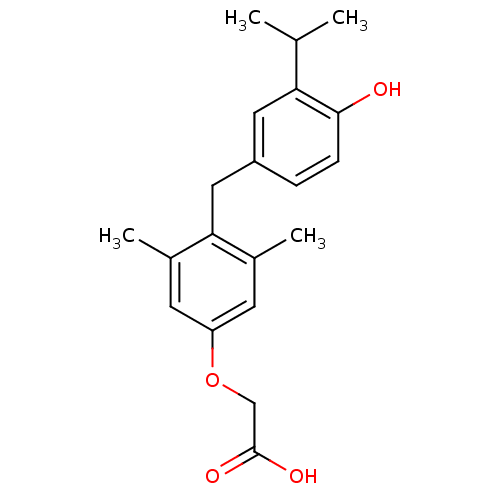
(3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...)Show InChI InChI=1S/C20H24O4/c1-12(2)17-9-15(5-6-19(17)21)10-18-13(3)7-16(8-14(18)4)24-11-20(22)23/h5-9,12,21H,10-11H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a |
OREGON HEALTH & SCIENCE UNIVERSITY
US Patent
| Assay Description
Human epithelial kidney cells (HEK 293) were grown to 80% confluency in Dubelcco's modified Eagles 4.5 g/L glucose medium (high glucose DMEM) con... |
US Patent US10544075 (2020)
BindingDB Entry DOI: 10.7270/Q2NP26SZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18920
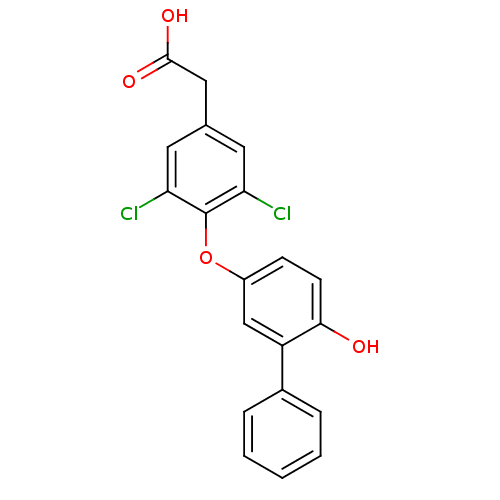
(2-[3,5-dichloro-4-(4-hydroxy-3-phenylphenoxy)pheny...)Show SMILES OC(=O)Cc1cc(Cl)c(Oc2ccc(O)c(c2)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C20H14Cl2O4/c21-16-8-12(10-19(24)25)9-17(22)20(16)26-14-6-7-18(23)15(11-14)13-4-2-1-3-5-13/h1-9,11,23H,10H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity to TRbeta1 |
J Med Chem 55: 5649-75 (2012)
Article DOI: 10.1021/jm2004706
BindingDB Entry DOI: 10.7270/Q2DZ09FJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605644

(CHEMBL5209093)Show SMILES OC(=O)CNC(=O)c1nc(Br)c2cc(Oc3ccccc3)ccc2c1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178971
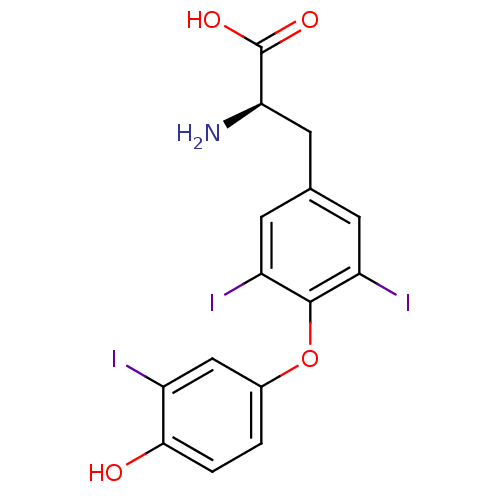
((R)-2-Amino-3-[4-(4-hydroxy-3-iodo-phenoxy)-3,5-di...)Show SMILES N[C@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Efficacy against TRAF-beta-1 reporter cells |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | 3.5 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
J Med Chem 46: 1580-8 (2003)
Article DOI: 10.1021/jm021080f
BindingDB Entry DOI: 10.7270/Q20K26TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178974
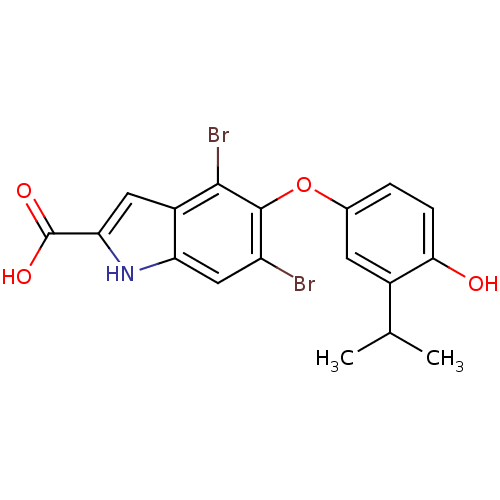
(4,6-dibromo-5-(4-hydroxy-3-isopropylphenoxy)-1H-in...)Show SMILES CC(C)c1cc(Oc2c(Br)cc3[nH]c(cc3c2Br)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Br2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Efficacy against TRAF-beta-1 reporter cells |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178972
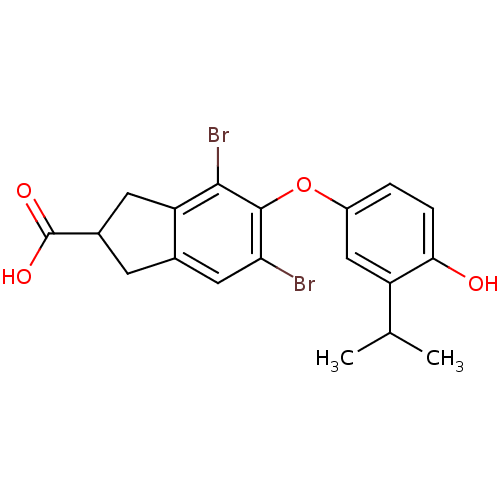
(4,6-dibromo-5-(4-hydroxy-3-isopropylphenoxy)-2,3-d...)Show SMILES CC(C)c1cc(Oc2c(Br)cc3CC(Cc3c2Br)C(O)=O)ccc1O Show InChI InChI=1S/C19H18Br2O4/c1-9(2)13-8-12(3-4-16(13)22)25-18-15(20)7-10-5-11(19(23)24)6-14(10)17(18)21/h3-4,7-9,11,22H,5-6H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Efficacy against TRAF-beta-1 reporter cells |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50178975
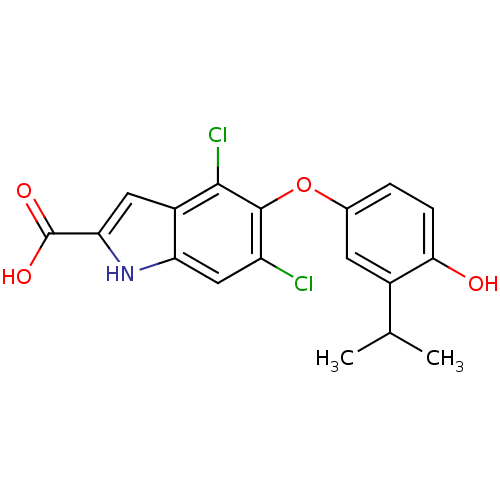
(4,6-dichloro-5-(4-hydroxy-3-isopropylphenoxy)-1H-i...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc3[nH]c(cc3c2Cl)C(O)=O)ccc1O Show InChI InChI=1S/C18H15Cl2NO4/c1-8(2)10-5-9(3-4-15(10)22)25-17-12(19)7-13-11(16(17)20)6-14(21-13)18(23)24/h3-8,21-22H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Karo Bio AB
Curated by ChEMBL
| Assay Description
Efficacy against TRAF-beta-1 reporter cells |
Bioorg Med Chem Lett 16: 1240-4 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.077
BindingDB Entry DOI: 10.7270/Q2T72H1W |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605642
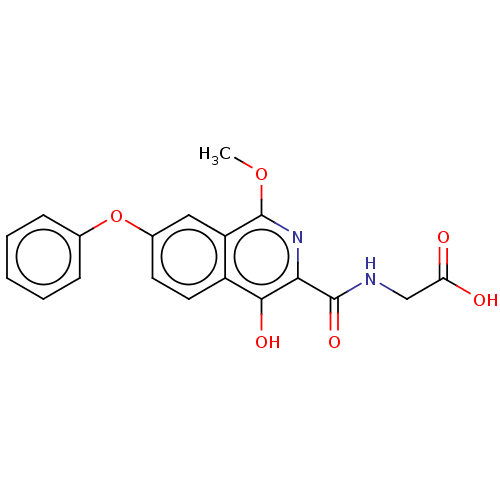
(CHEMBL5172205)Show SMILES COc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059676, Compound ZB-H-54
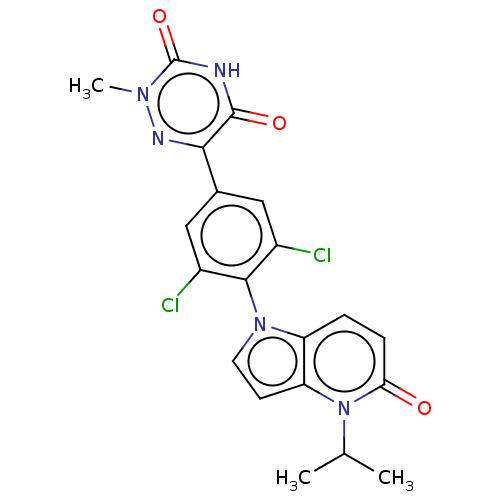
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605651
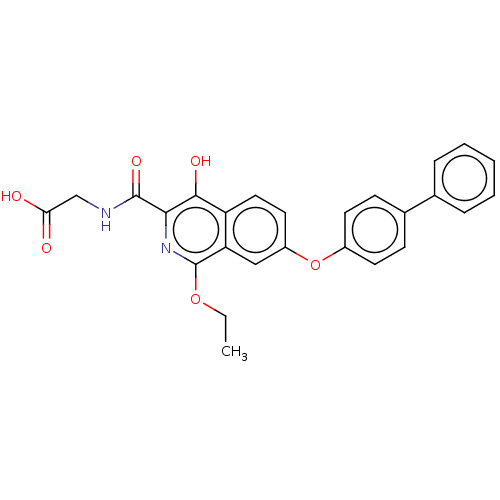
(CHEMBL5186156)Show SMILES CCOc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccc(cc3)-c3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605655
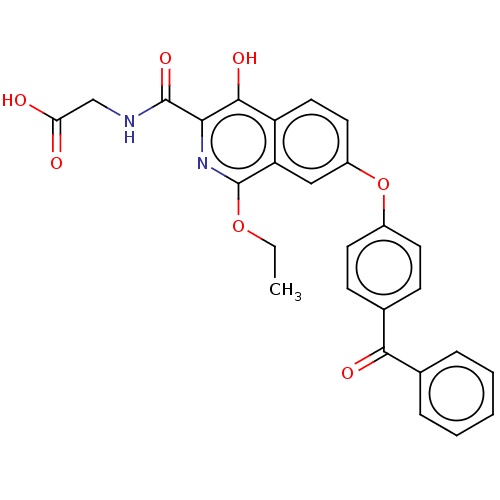
(CHEMBL5207186)Show SMILES CCOc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccc(cc3)C(=O)c3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50115668

(3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...)Show InChI InChI=1S/C20H24O4/c1-12(2)17-9-15(5-6-19(17)21)10-18-13(3)7-16(8-14(18)4)24-11-20(22)23/h5-9,12,21H,10-11H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Half-maximum activation of human Thyroid hormone receptor beta 1 (hTRbeta1) |
J Med Chem 45: 3310-20 (2002)
BindingDB Entry DOI: 10.7270/Q20G3JHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM466857

(US10800767, Example 1 | US10800767, Example 16)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(NCc3noc(=O)[nH]3)cc2Cl)nn(C)c1=O Show InChI InChI=1S/C17H17Cl2N5O4/c1-8(2)10-6-14(22-24(3)16(10)25)27-15-11(18)4-9(5-12(15)19)20-7-13-21-17(26)28-23-13/h4-6,8,20H,7H2,1-3H3,(H,21,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
Terns, Inc.
US Patent
| Assay Description
LanthaScreen TR-FRET Thyroid Receptor alpha Coactivator Assay kit (ThermoFisher) and LanthaScreen™ TR-FRET Thyroid Receptor beta Coactivator Assay ki... |
US Patent US10800767 (2020)
BindingDB Entry DOI: 10.7270/Q25X2D1N |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18873
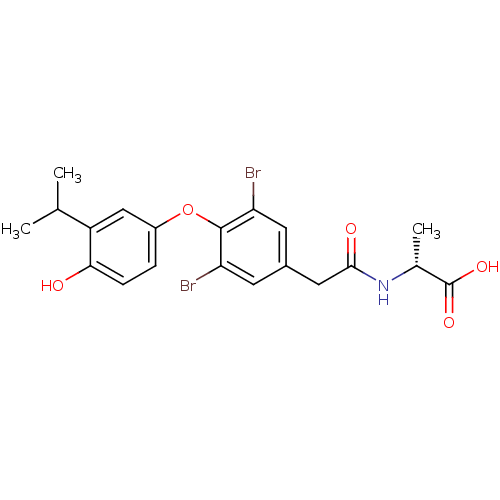
((2R)-2-(1-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(=O)N[C@H](C)C(O)=O)cc2Br)ccc1O |r| Show InChI InChI=1S/C20H21Br2NO5/c1-10(2)14-9-13(4-5-17(14)24)28-19-15(21)6-12(7-16(19)22)8-18(25)23-11(3)20(26)27/h4-7,9-11,24H,8H2,1-3H3,(H,23,25)(H,26,27)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | 7.60 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605654
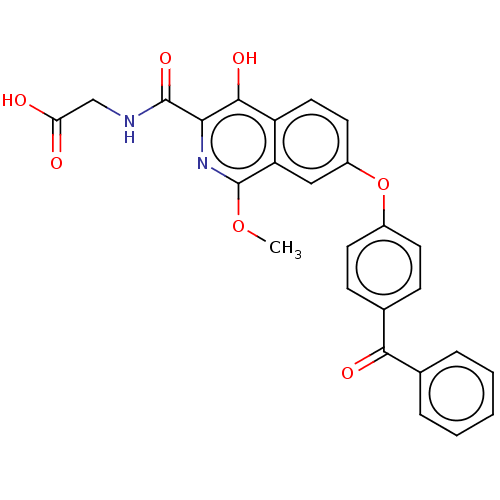
(CHEMBL5179829)Show SMILES COc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccc(cc3)C(=O)c3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605650
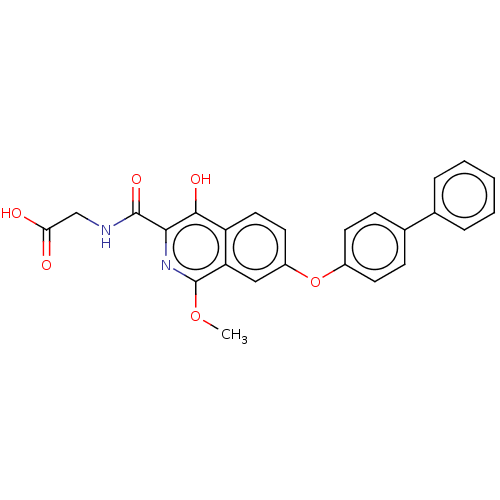
(CHEMBL5171808)Show SMILES COc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccc(cc3)-c3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | CHEMBL5267199

| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605658
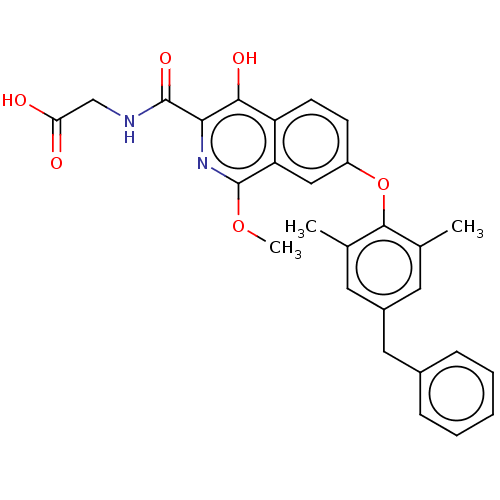
(CHEMBL5188839)Show SMILES COc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3c(C)cc(Cc4ccccc4)cc3C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00641
BindingDB Entry DOI: 10.7270/Q2K35ZR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | US20240059676, Compound ZB-H-18
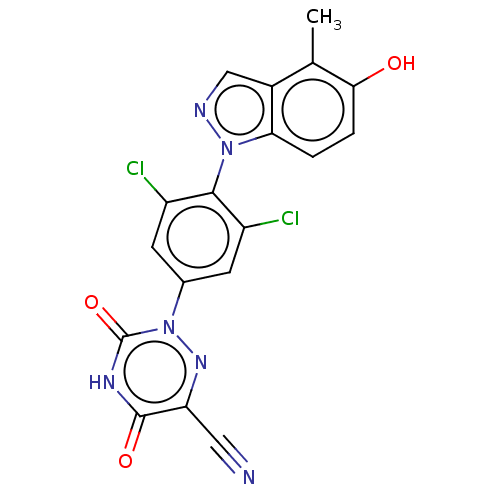
| PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of California-San Francisco
| Assay Description
TRE-driven dual-luciferase reporter assay in human bone osteosarcoma epithelial (U2OS) cell line. |
ACS Chem Biol 1: 585-93 (2006)
Article DOI: 10.1021/cb600311v
BindingDB Entry DOI: 10.7270/Q23N21R4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of California-San Francisco
Curated by ChEMBL
| Assay Description
Effect on human TRbeta transactivation activity in U2OS cells by luciferase reporter assay |
Bioorg Med Chem 16: 762-70 (2008)
Article DOI: 10.1016/j.bmc.2007.10.040
BindingDB Entry DOI: 10.7270/Q2RR2034 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18880
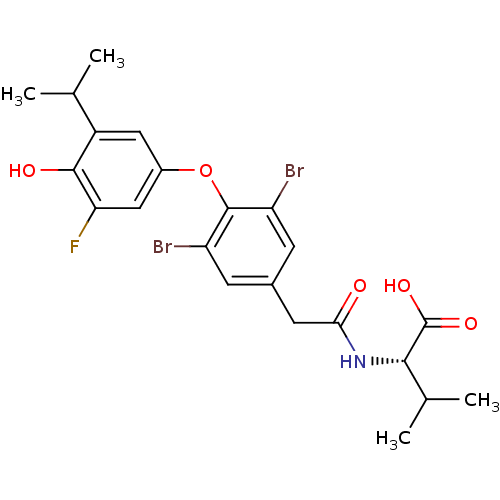
((2S)-2-(1-{3,5-dibromo-4-[3-fluoro-4-hydroxy-5-(pr...)Show SMILES CC(C)[C@H](NC(=O)Cc1cc(Br)c(Oc2cc(F)c(O)c(c2)C(C)C)c(Br)c1)C(O)=O |r| Show InChI InChI=1S/C22H24Br2FNO5/c1-10(2)14-8-13(9-17(25)20(14)28)31-21-15(23)5-12(6-16(21)24)7-18(27)26-19(11(3)4)22(29)30/h5-6,8-11,19,28H,7H2,1-4H3,(H,26,27)(H,29,30)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | 11 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... |
Bioorg Med Chem Lett 17: 4131-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.049
BindingDB Entry DOI: 10.7270/Q2VT1QB6 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50605656
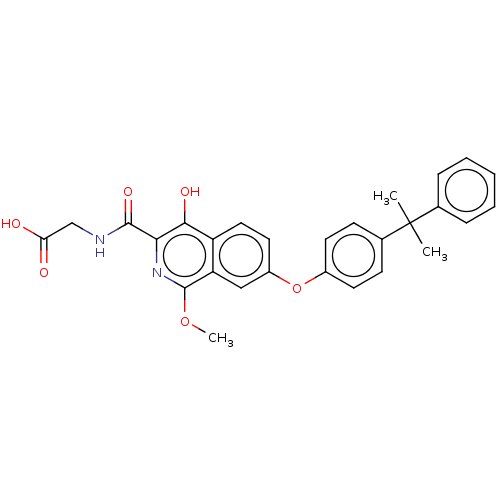
(CHEMBL5191021)Show SMILES COc1nc(C(=O)NCC(O)=O)c(O)c2ccc(Oc3ccc(cc3)C(C)(C)c3ccccc3)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00144
BindingDB Entry DOI: 10.7270/Q2ZS31MV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data