

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430798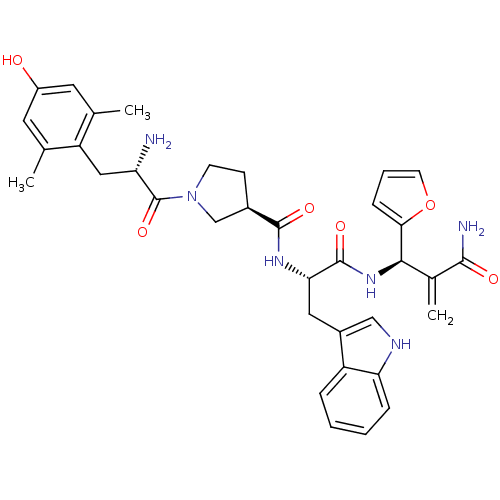 (CHEMBL2335120) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0000420 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430801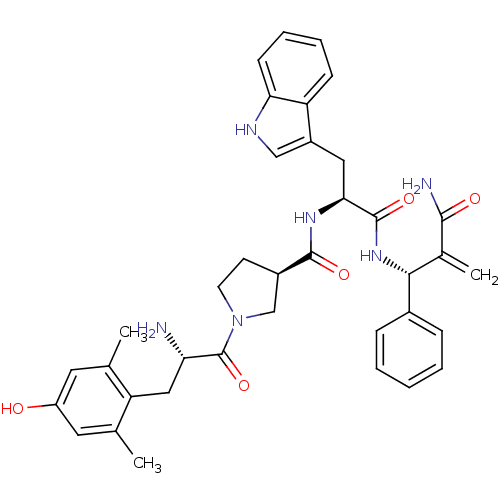 (CHEMBL2334776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0000910 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430803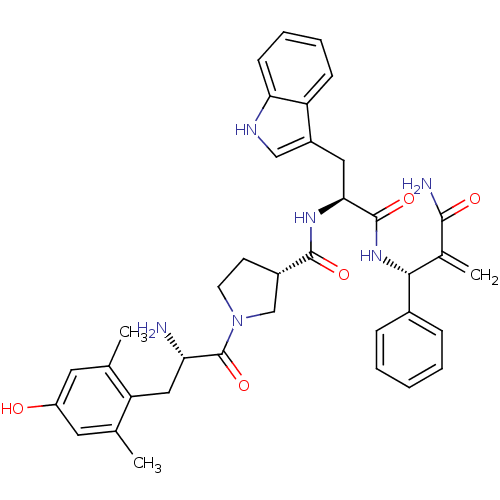 (CHEMBL2334774) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000107 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430802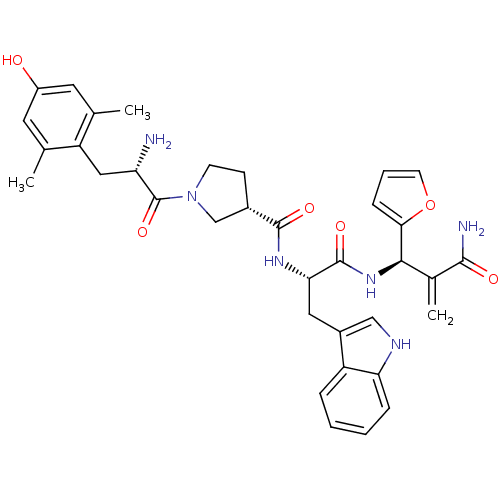 (CHEMBL2334775) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000109 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430799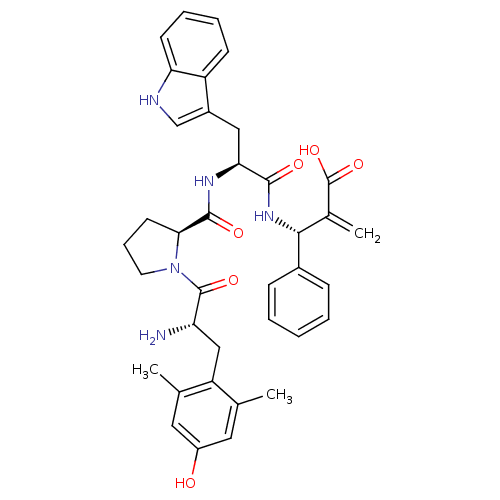 (CHEMBL2334772) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000130 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594825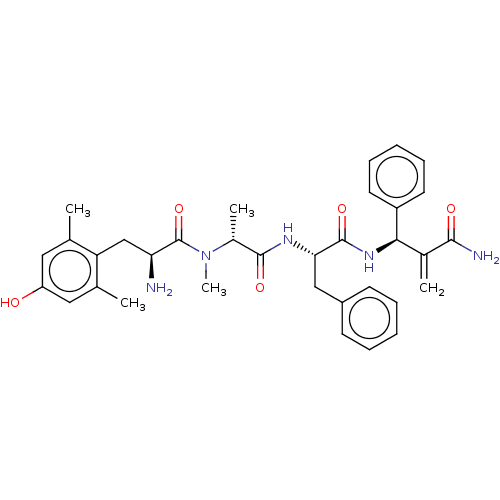 (CHEMBL5198856) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.000515 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430800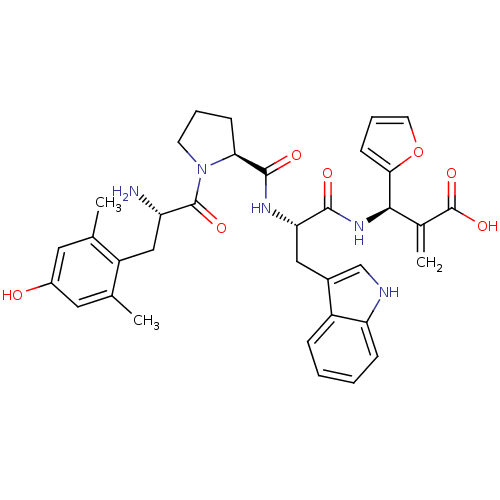 (CHEMBL2334773) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.000516 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50095155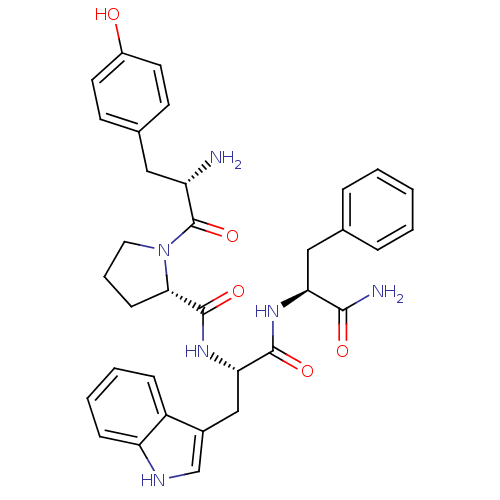 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Activity at mu opioid receptor assessed as increase in calcium level in CHO cells by aequorin luminescence based calcium assay | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50393257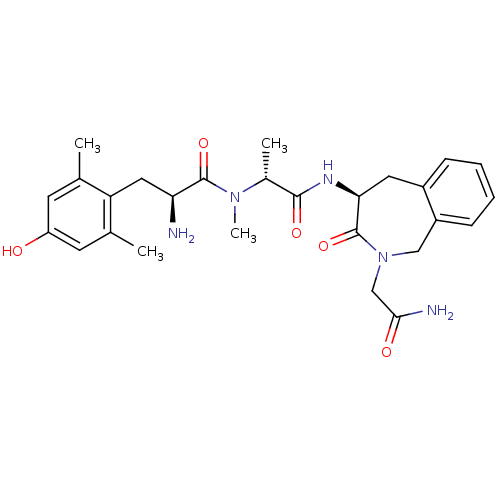 (CHEMBL2151735) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00174 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat... | J Med Chem 54: 7848-59 (2011) Article DOI: 10.1021/jm200894e BindingDB Entry DOI: 10.7270/Q25X2B1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528966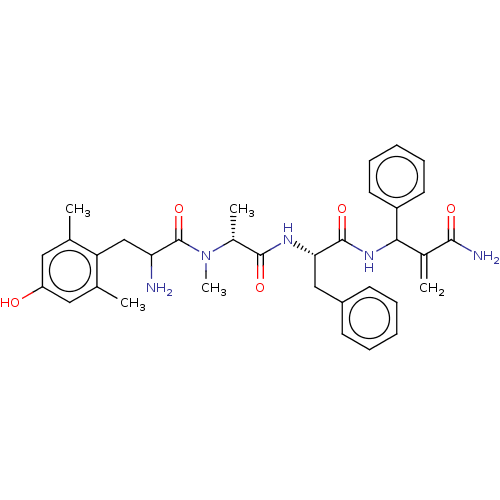 (CHEMBL4521879) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00177 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528965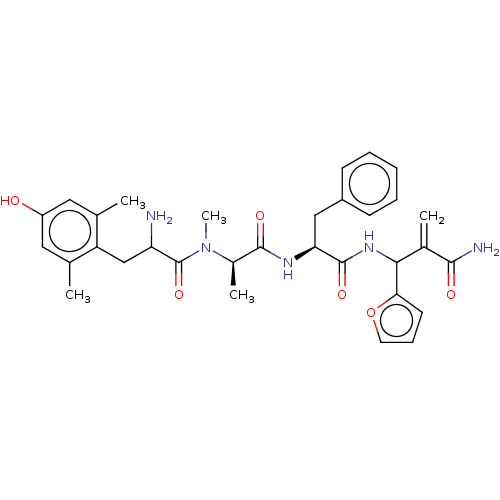 (CHEMBL4550234) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00177 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528960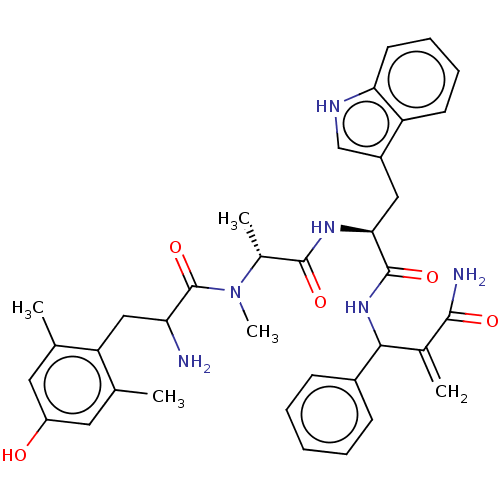 (CHEMBL4450250) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00177 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528967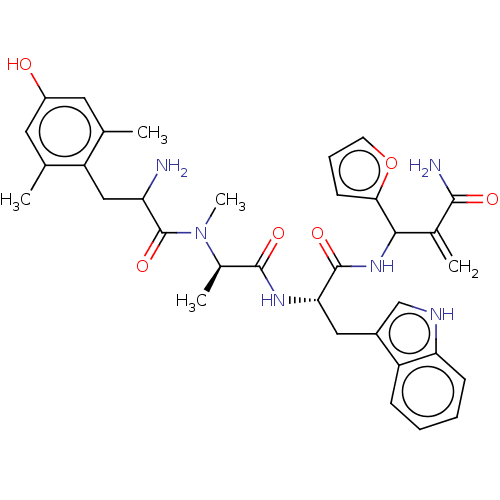 (CHEMBL4439415) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00177 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation counting | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594821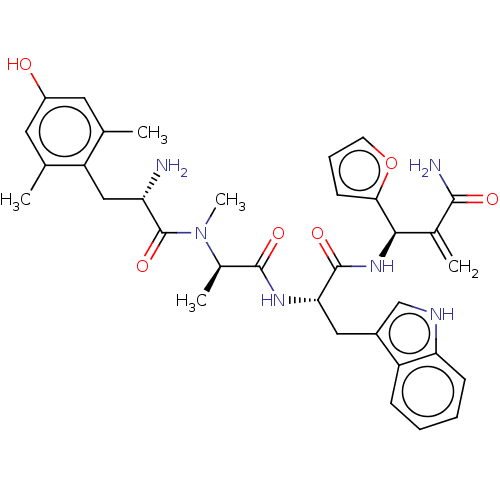 (CHEMBL5175179) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00180 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594822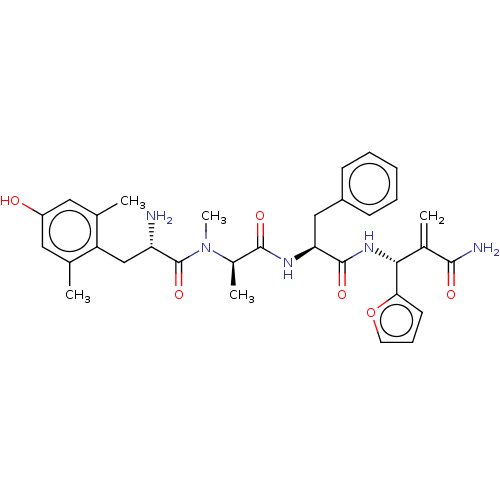 (CHEMBL5182849) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00201 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50529413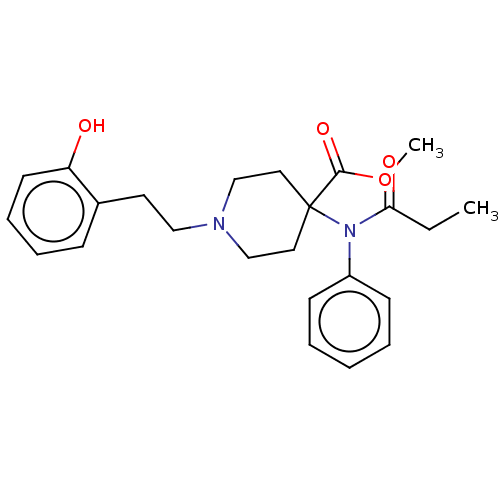 (CHEMBL4563672) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00240 | n/a | n/a | n/a | n/a |
United States Army CCDC Chemical Biological Center Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer... | ACS Med Chem Lett 10: 1568-1572 (2019) Article DOI: 10.1021/acsmedchemlett.9b00404 BindingDB Entry DOI: 10.7270/Q2JD5175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274679 (CHEMBL4128530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasma | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594820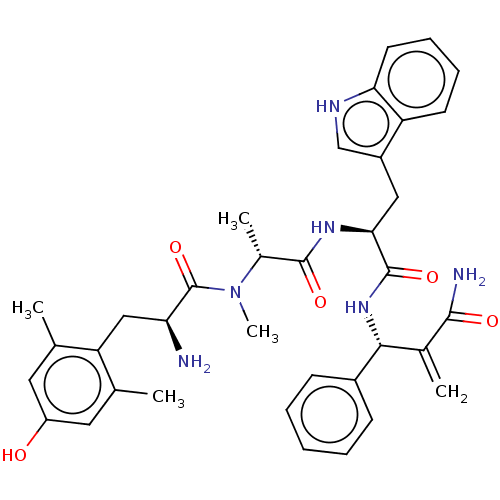 (CHEMBL5176887) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012477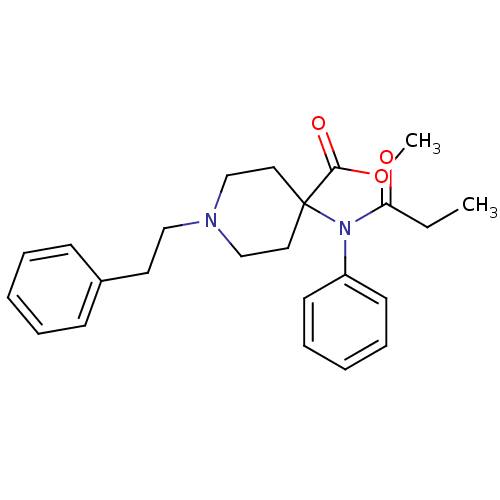 (1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00490 | n/a | n/a | n/a | n/a |
United States Army CCDC Chemical Biological Center Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer... | ACS Med Chem Lett 10: 1568-1572 (2019) Article DOI: 10.1021/acsmedchemlett.9b00404 BindingDB Entry DOI: 10.7270/Q2JD5175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50529410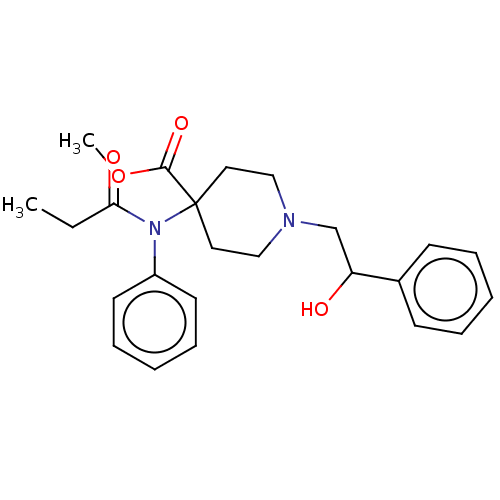 (CHEMBL4463749) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00510 | n/a | n/a | n/a | n/a |
United States Army CCDC Chemical Biological Center Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer... | ACS Med Chem Lett 10: 1568-1572 (2019) Article DOI: 10.1021/acsmedchemlett.9b00404 BindingDB Entry DOI: 10.7270/Q2JD5175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274694 (CHEMBL4129901) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274686 (CHEMBL4129679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430806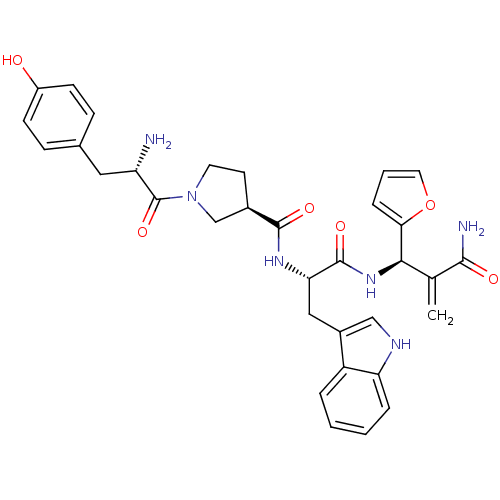 (CHEMBL2334771) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00853 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274689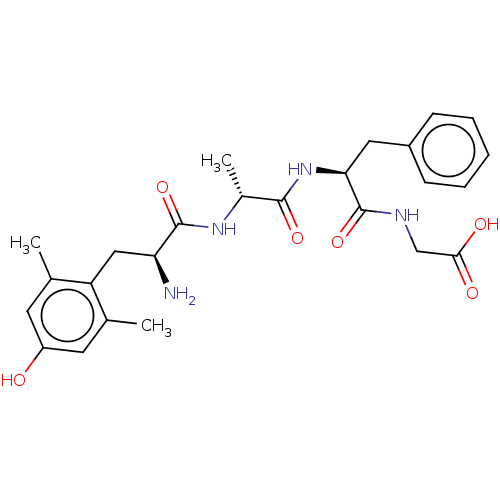 (CHEMBL4126050) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274696 (CHEMBL4126102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50529415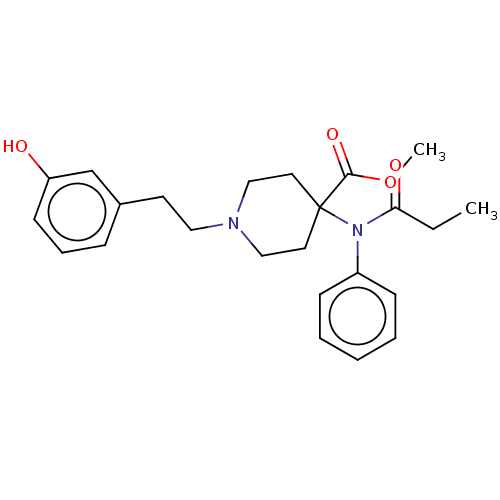 (CHEMBL4588535) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a |
United States Army CCDC Chemical Biological Center Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer... | ACS Med Chem Lett 10: 1568-1572 (2019) Article DOI: 10.1021/acsmedchemlett.9b00404 BindingDB Entry DOI: 10.7270/Q2JD5175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50550066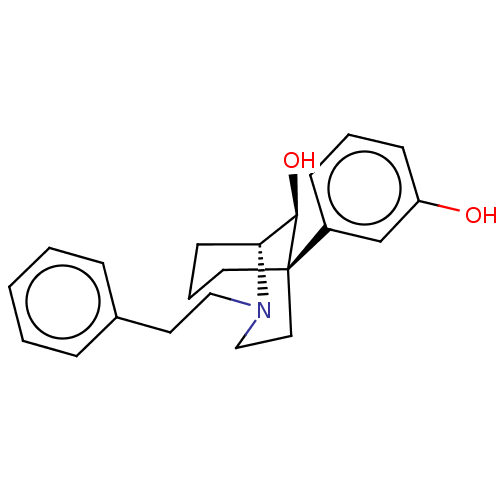 (CHEMBL4798954) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human MOR expressed in CHOK1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation incubated for 30 mins by lumi... | Citation and Details Article DOI: 10.1039/d0md00104j BindingDB Entry DOI: 10.7270/Q2K64NQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50529414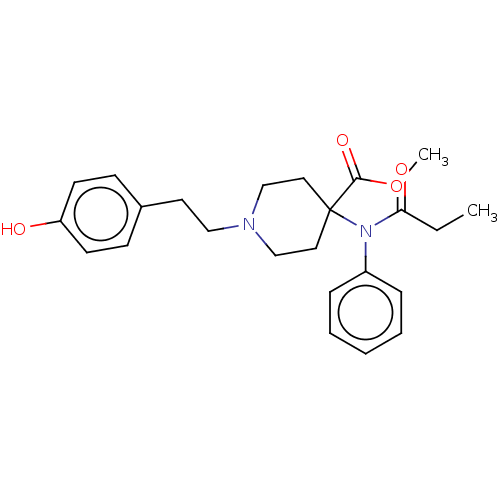 (CHEMBL4578287) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a |
United States Army CCDC Chemical Biological Center Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in CHO-K1 cells assessed as cAMP accumulation incubated for 30 mins and measured after 1 hr by Eu-cAMP tracer... | ACS Med Chem Lett 10: 1568-1572 (2019) Article DOI: 10.1021/acsmedchemlett.9b00404 BindingDB Entry DOI: 10.7270/Q2JD5175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50594865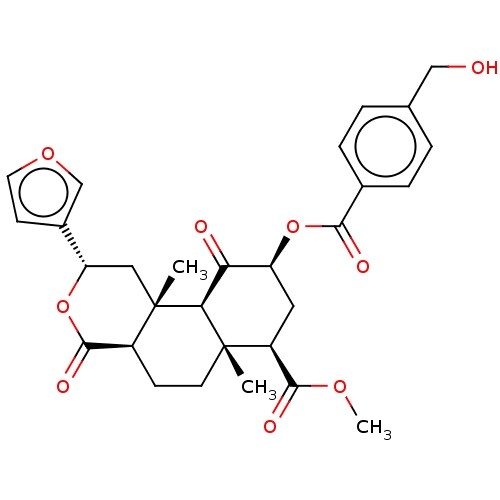 (CHEMBL5186887) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01915 BindingDB Entry DOI: 10.7270/Q22B931S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50395060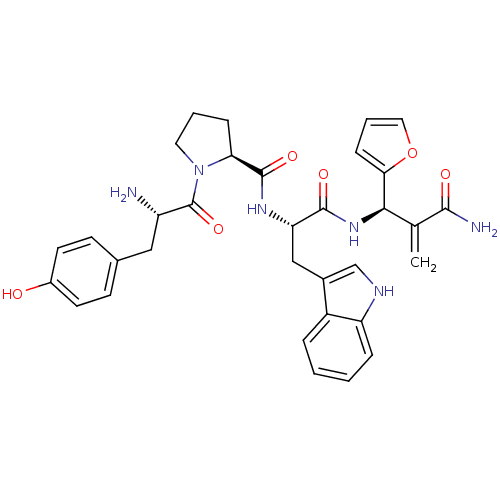 (CHEMBL2163916) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0334 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins by liqui... | J Med Chem 55: 6224-36 (2012) Article DOI: 10.1021/jm300664y BindingDB Entry DOI: 10.7270/Q2T154S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50395059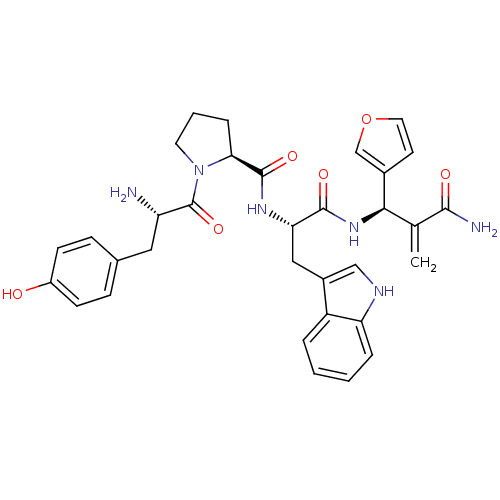 (CHEMBL2163917) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0342 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins by liqui... | J Med Chem 55: 6224-36 (2012) Article DOI: 10.1021/jm300664y BindingDB Entry DOI: 10.7270/Q2T154S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430807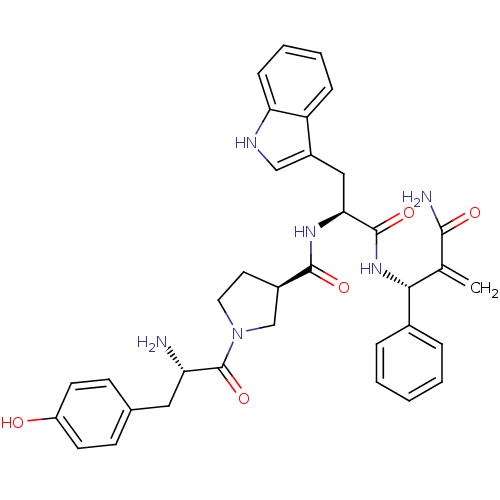 (CHEMBL2334770) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0397 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM518593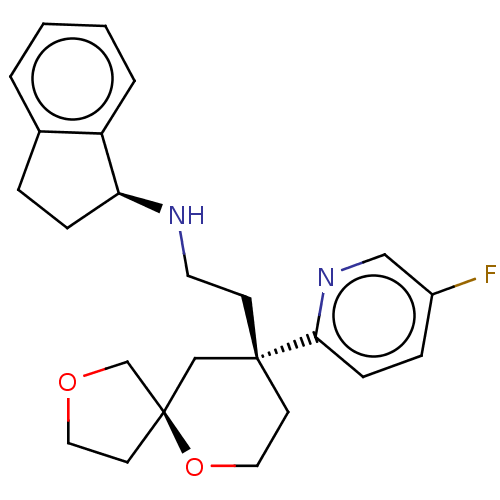 (US11124523, Example (+)-28b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA | Assay Description The experiment was performed using a cAMP detection kit from Cisbio (Cisbio #62AM4PEJ). | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274697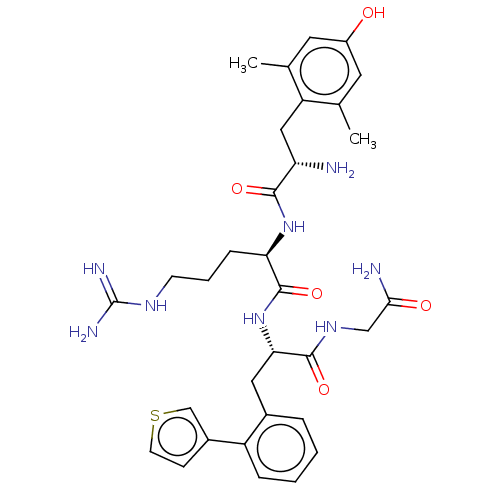 (CHEMBL4129689) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027230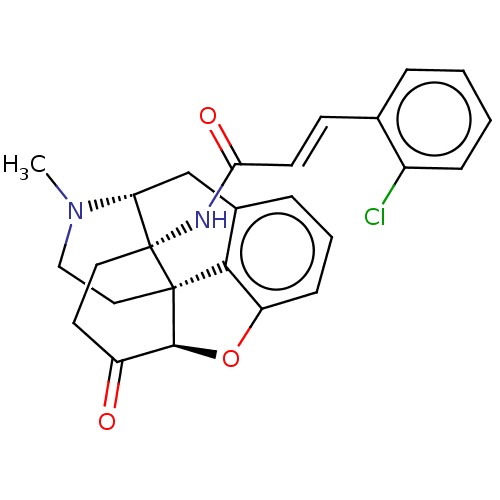 (CHEMBL2113666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Stimulation of [35S]GTPgammaS binding to human recombinant MOR | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139013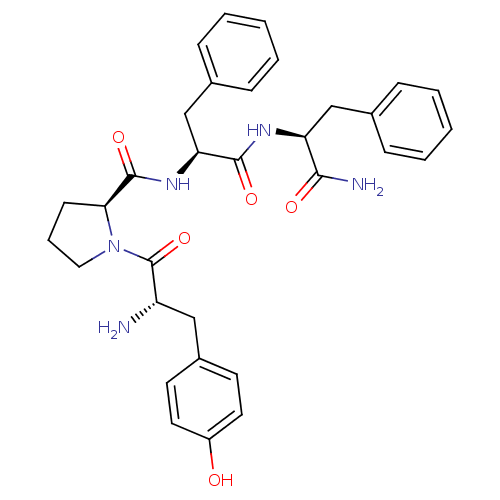 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Antagonist activity at mu opioid receptor expressed in CHO cells assessed as release of intracellular calcium ions by aequorin luminescence-based cal... | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50193546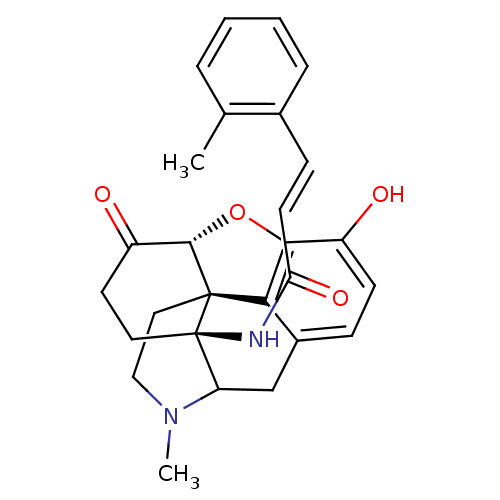 (14beta-(2'-methylcinnamoylamino)-7,8-dihydromorphi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Stimulation of [35S]GTPgammaS binding to human recombinant MOR | J Med Chem 49: 5333-8 (2006) Article DOI: 10.1021/jm0604777 BindingDB Entry DOI: 10.7270/Q2WH2QSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Medical University Curated by ChEMBL | Assay Description Activity at mu opioid receptor assessed as increase in calcium level in CHO cells by aequorin luminescence based calcium assay | J Med Chem 50: 512-20 (2007) Article DOI: 10.1021/jm060998u BindingDB Entry DOI: 10.7270/Q28915JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430805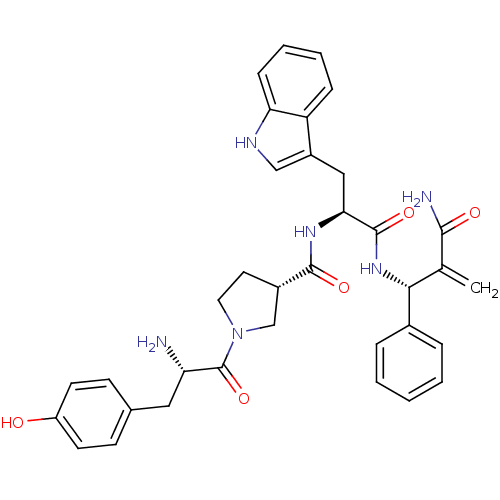 (CHEMBL2334768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAM... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50127700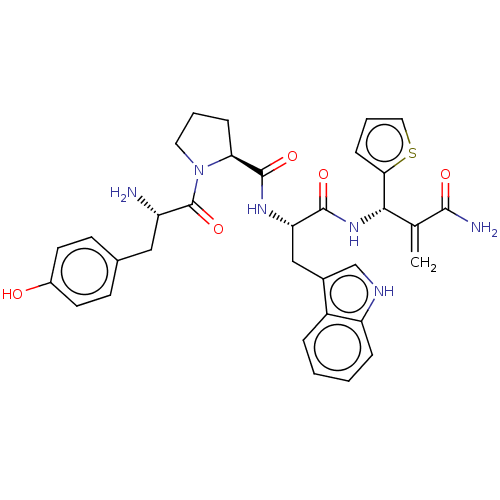 (CHEMBL3629337) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0629 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor (unknown origin) transfected in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 5393-7 (2015) Article DOI: 10.1016/j.bmcl.2015.09.025 BindingDB Entry DOI: 10.7270/Q24M96CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM397187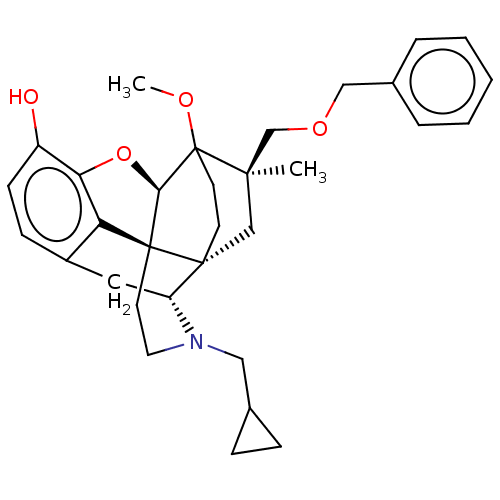 (US9988392, Compound 7) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals | Assay Description μ-Opioid:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.),... | Bioorg Med Chem 17: 3934-58 (2009) BindingDB Entry DOI: 10.7270/Q28K7CF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50127701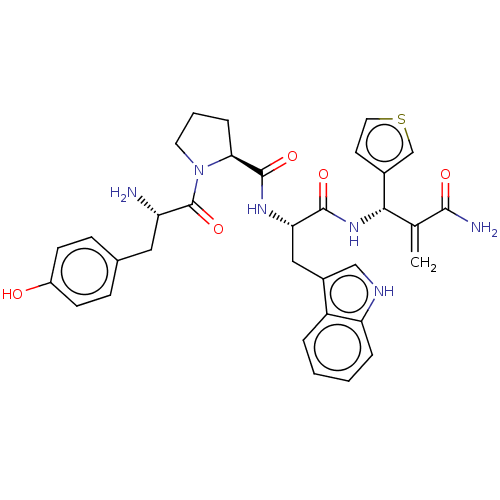 (CHEMBL3627737) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0643 | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor (unknown origin) transfected in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 5393-7 (2015) Article DOI: 10.1016/j.bmcl.2015.09.025 BindingDB Entry DOI: 10.7270/Q24M96CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50583967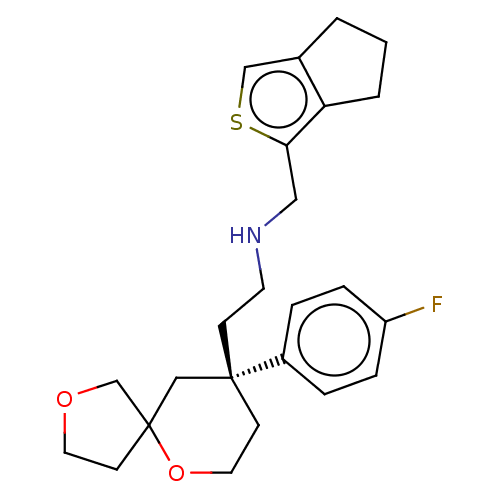 (CHEMBL5074742) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at mu opioid receptor (unknown origin) assessed as increase in cAMP level incubated for 40 mins by spectrophotometry | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113986 BindingDB Entry DOI: 10.7270/Q2TX3K84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM518565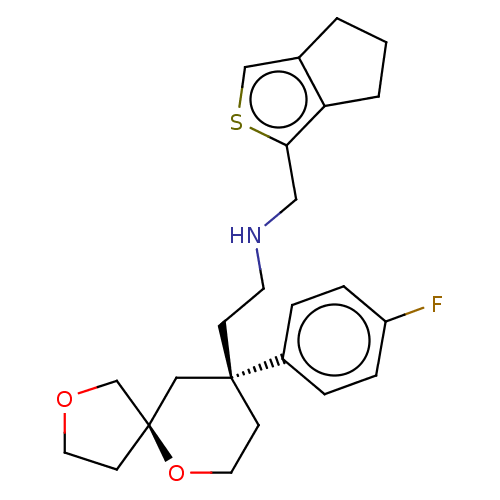 (US11124523, Example (+)-4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
TBA | Assay Description The experiment was performed using a cAMP detection kit from Cisbio (Cisbio #62AM4PEJ). | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017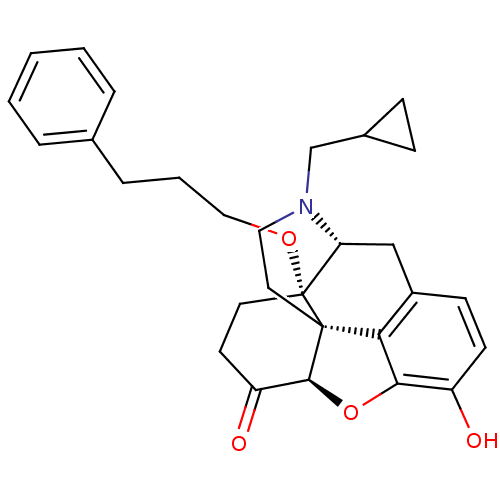 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50393258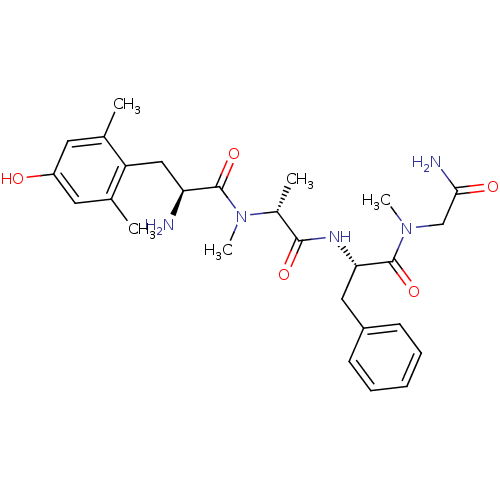 (CHEMBL2151734) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat... | J Med Chem 54: 7848-59 (2011) Article DOI: 10.1021/jm200894e BindingDB Entry DOI: 10.7270/Q25X2B1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354650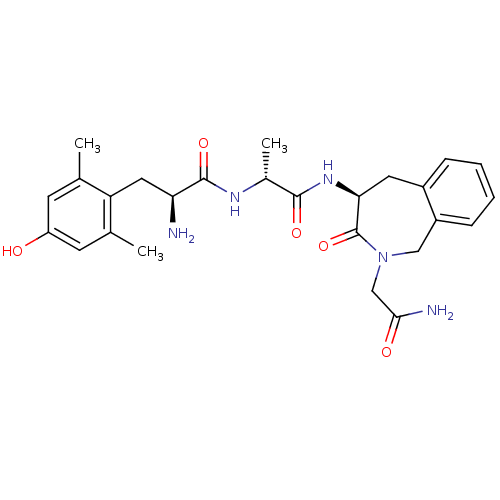 (CHEMBL1834247) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0832 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cell membrane assessed as inhibition of forskolin-induced cAMP accumulation by by liquid scintillat... | J Med Chem 54: 7848-59 (2011) Article DOI: 10.1021/jm200894e BindingDB Entry DOI: 10.7270/Q25X2B1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3234 total ) | Next | Last >> |