 Found 20 hits of ec50 data for polymerid = 2103,2104
Found 20 hits of ec50 data for polymerid = 2103,2104 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50413782
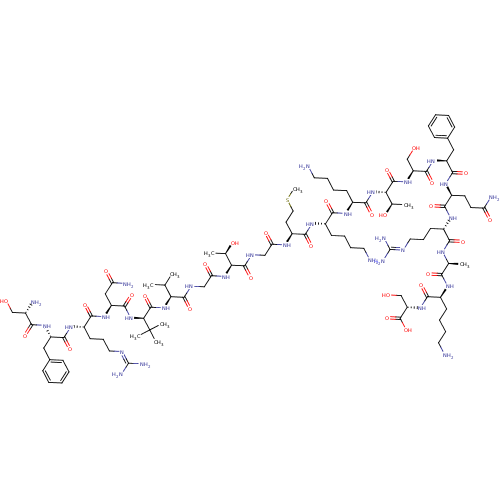
(CHEMBL1627325)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])C([#6])([#6])[#6])-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C97H163N31O28S/c1-50(2)73(126-93(154)76(97(6,7)8)128-88(149)66(44-70(103)135)122-81(142)61(32-23-40-109-96(106)107)118-86(147)64(120-78(139)56(101)47-129)42-54-24-12-10-13-25-54)90(151)110-46-72(137)125-74(52(4)132)91(152)111-45-71(136)113-63(35-41-157-9)84(145)115-58(29-17-20-37-99)80(141)116-59(30-18-21-38-100)85(146)127-75(53(5)133)92(153)123-67(48-130)89(150)121-65(43-55-26-14-11-15-27-55)87(148)119-62(33-34-69(102)134)83(144)117-60(31-22-39-108-95(104)105)79(140)112-51(3)77(138)114-57(28-16-19-36-98)82(143)124-68(49-131)94(155)156/h10-15,24-27,50-53,56-68,73-76,129-133H,16-23,28-49,98-101H2,1-9H3,(H2,102,134)(H2,103,135)(H,110,151)(H,111,152)(H,112,140)(H,113,136)(H,114,138)(H,115,145)(H,116,141)(H,117,144)(H,118,147)(H,119,148)(H,120,139)(H,121,150)(H,122,142)(H,123,153)(H,124,143)(H,125,137)(H,126,154)(H,127,146)(H,128,149)(H,155,156)(H4,104,105,108)(H4,106,107,109)/t51-,52+,53+,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,73-,74-,75-,76-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a |
National Institute of Neuroscienc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NK1 receptor expressed in CHO cells assessed as inhibition of NPS-induced intracellular calcium mobilization |
J Med Chem 52: 4068-71 (2009)
Article DOI: 10.1021/jm900604g
BindingDB Entry DOI: 10.7270/Q2445NQD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0575 | n/a | n/a | n/a | n/a |
National Institute of Neuroscienc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NK1 receptor expressed in CHO cells assessed as inhibition of NPS-induced intracellular calcium mobilization |
J Med Chem 52: 4068-71 (2009)
Article DOI: 10.1021/jm900604g
BindingDB Entry DOI: 10.7270/Q2445NQD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche AG
Curated by ChEMBL
| Assay Description
Agonist activity at wild type human NK1 receptor expressed in HEK293 cells assessed as increase in [3H]IP accumulation after 20 mins |
J Med Chem 55: 5061-76 (2012)
Article DOI: 10.1021/jm2017072
BindingDB Entry DOI: 10.7270/Q2959JP4 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087851

(CHEMBL384518 | LysProSerProAspArgPheTyrGlyLeuMet)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN)C(N)=O Show InChI InChI=1S/C60H92N16O15S/c1-34(2)28-41(53(85)69-39(50(63)82)22-27-92-3)68-48(79)32-67-51(83)42(30-36-18-20-37(78)21-19-36)71-54(86)43(29-35-12-5-4-6-13-35)72-52(84)40(15-9-24-66-60(64)65)70-55(87)44(31-49(80)81)73-56(88)47-17-11-26-76(47)59(91)45(33-77)74-57(89)46-16-10-25-75(46)58(90)38(62)14-7-8-23-61/h4-6,12-13,18-21,34,38-47,77-78H,7-11,14-17,22-33,61-62H2,1-3H3,(H2,63,82)(H,67,83)(H,68,79)(H,69,85)(H,70,87)(H,71,86)(H,72,84)(H,73,88)(H,74,89)(H,80,81)(H4,64,65,66)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at bfSPR to produce increase in intracellular [Ca2+] |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
SP-induced [Ca2+] mobilization in CHO cells expressing human Tachykinin receptor 1 |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087851

(CHEMBL384518 | LysProSerProAspArgPheTyrGlyLeuMet)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN)C(N)=O Show InChI InChI=1S/C60H92N16O15S/c1-34(2)28-41(53(85)69-39(50(63)82)22-27-92-3)68-48(79)32-67-51(83)42(30-36-18-20-37(78)21-19-36)71-54(86)43(29-35-12-5-4-6-13-35)72-52(84)40(15-9-24-66-60(64)65)70-55(87)44(31-49(80)81)73-56(88)47-17-11-26-76(47)59(91)45(33-77)74-57(89)46-16-10-25-75(46)58(90)38(62)14-7-8-23-61/h4-6,12-13,18-21,34,38-47,77-78H,7-11,14-17,22-33,61-62H2,1-3H3,(H2,63,82)(H,67,83)(H,68,79)(H,69,85)(H,70,87)(H,71,86)(H,72,84)(H,73,88)(H,74,89)(H,80,81)(H4,64,65,66)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
SP-induced [Ca2+] mobilization in CHO cells expressing human Tachykinin receptor 1 |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at bfSPR to produce increase in intracellular [Ca2+] |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030211
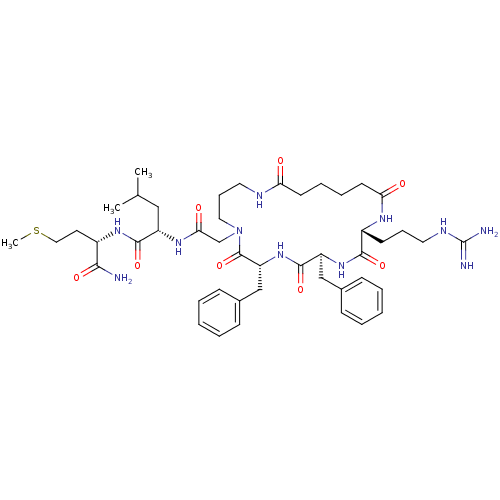
((S)-2-{2-[(2R,5S,8R)-5,8-Dibenzyl-2-(3-guanidino-p...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C46H69N11O8S/c1-30(2)26-35(43(63)54-33(41(47)61)21-25-66-3)53-40(60)29-57-24-13-23-50-38(58)19-10-11-20-39(59)52-34(18-12-22-51-46(48)49)42(62)55-36(27-31-14-6-4-7-15-31)44(64)56-37(45(57)65)28-32-16-8-5-9-17-32/h4-9,14-17,30,33-37H,10-13,18-29H2,1-3H3,(H2,47,61)(H,50,58)(H,52,59)(H,53,60)(H,54,63)(H,55,62)(H,56,64)(H4,48,49,51)/t33-,34+,35-,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Technische Universität München
Curated by ChEMBL
| Assay Description
Tested for the biological activity and selectivity against NK-1 receptor |
J Med Chem 37: 2145-52 (1994)
BindingDB Entry DOI: 10.7270/Q2NG4PNN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030213
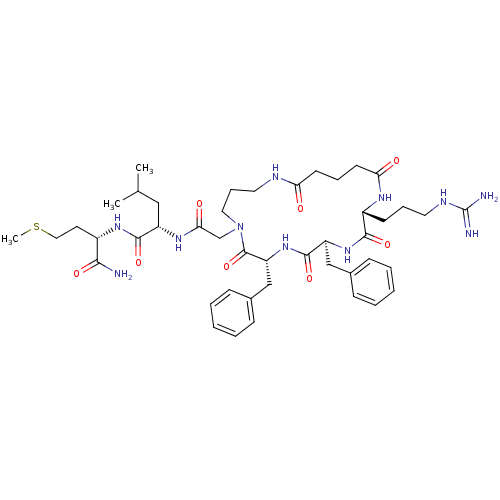
((S)-2-{2-[(2R,5S,8R)-5,8-Dibenzyl-2-(3-guanidino-p...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CN1CCCNC(=O)CCCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C45H67N11O8S/c1-29(2)25-34(42(62)53-32(40(46)60)20-24-65-3)52-39(59)28-56-23-12-22-49-37(57)18-10-19-38(58)51-33(17-11-21-50-45(47)48)41(61)54-35(26-30-13-6-4-7-14-30)43(63)55-36(44(56)64)27-31-15-8-5-9-16-31/h4-9,13-16,29,32-36H,10-12,17-28H2,1-3H3,(H2,46,60)(H,49,57)(H,51,58)(H,52,59)(H,53,62)(H,54,61)(H,55,63)(H4,47,48,50)/t32-,33+,34-,35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Technische Universität München
Curated by ChEMBL
| Assay Description
Tested for the biological activity and selectivity against NK-1 receptor |
J Med Chem 37: 2145-52 (1994)
BindingDB Entry DOI: 10.7270/Q2NG4PNN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087852
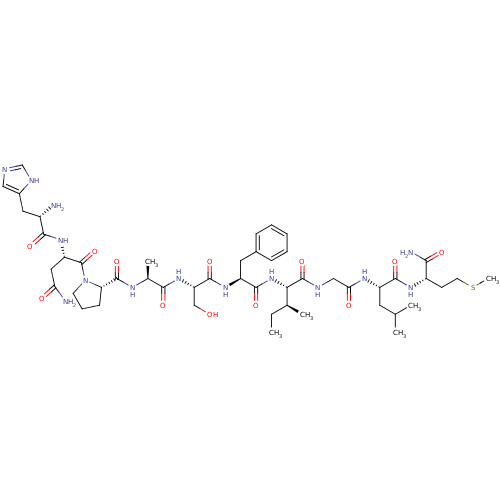
(CHEMBL263185 | His-Asn-Pro-Ala-Ser-Phe-Ile-Gly-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O Show InChI InChI=1S/C49H76N14O12S/c1-7-27(4)40(48(74)54-23-39(66)57-33(18-26(2)3)44(70)58-32(41(52)67)15-17-76-6)62-45(71)34(19-29-12-9-8-10-13-29)59-46(72)36(24-64)61-42(68)28(5)56-47(73)37-14-11-16-63(37)49(75)35(21-38(51)65)60-43(69)31(50)20-30-22-53-25-55-30/h8-10,12-13,22,25-28,31-37,40,64H,7,11,14-21,23-24,50H2,1-6H3,(H2,51,65)(H2,52,67)(H,53,55)(H,54,74)(H,56,73)(H,57,66)(H,58,70)(H,59,72)(H,60,69)(H,61,68)(H,62,71)/t27-,28-,31-,32-,33-,34-,35-,36-,37-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at bfSPR to produce increase in intracellular [Ca2+] |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087852

(CHEMBL263185 | His-Asn-Pro-Ala-Ser-Phe-Ile-Gly-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O Show InChI InChI=1S/C49H76N14O12S/c1-7-27(4)40(48(74)54-23-39(66)57-33(18-26(2)3)44(70)58-32(41(52)67)15-17-76-6)62-45(71)34(19-29-12-9-8-10-13-29)59-46(72)36(24-64)61-42(68)28(5)56-47(73)37-14-11-16-63(37)49(75)35(21-38(51)65)60-43(69)31(50)20-30-22-53-25-55-30/h8-10,12-13,22,25-28,31-37,40,64H,7,11,14-21,23-24,50H2,1-6H3,(H2,51,65)(H2,52,67)(H,53,55)(H,54,74)(H,56,73)(H,57,66)(H,58,70)(H,59,72)(H,60,69)(H,61,68)(H,62,71)/t27-,28-,31-,32-,33-,34-,35-,36-,37-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
SP-induced [Ca2+] mobilization in CHO cells expressing human Tachykinin receptor 1 |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087850
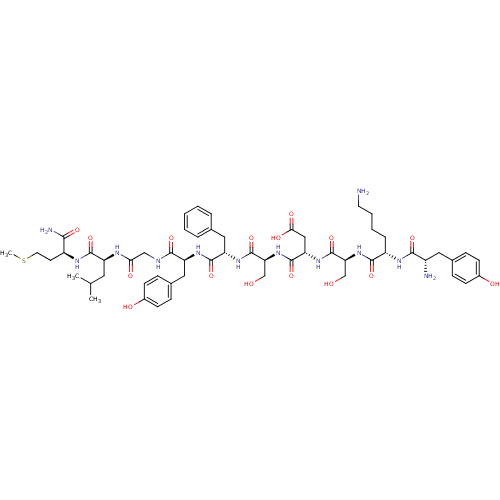
(CHEMBL415159 | TyrLysSerAspSerPheTyrGlyLeuMet)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C56H80N12O16S/c1-31(2)23-40(52(80)62-38(48(59)76)20-22-85-3)61-46(73)28-60-50(78)41(26-34-14-18-36(72)19-15-34)64-53(81)42(25-32-9-5-4-6-10-32)65-55(83)45(30-70)68-54(82)43(27-47(74)75)66-56(84)44(29-69)67-51(79)39(11-7-8-21-57)63-49(77)37(58)24-33-12-16-35(71)17-13-33/h4-6,9-10,12-19,31,37-45,69-72H,7-8,11,20-30,57-58H2,1-3H3,(H2,59,76)(H,60,78)(H,61,73)(H,62,80)(H,63,77)(H,64,81)(H,65,83)(H,66,84)(H,67,79)(H,68,82)(H,74,75)/t37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
SP-induced [Ca2+] mobilization in CHO cells expressing human Tachykinin receptor 1 |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50087850

(CHEMBL415159 | TyrLysSerAspSerPheTyrGlyLeuMet)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C56H80N12O16S/c1-31(2)23-40(52(80)62-38(48(59)76)20-22-85-3)61-46(73)28-60-50(78)41(26-34-14-18-36(72)19-15-34)64-53(81)42(25-32-9-5-4-6-10-32)65-55(83)45(30-70)68-54(82)43(27-47(74)75)66-56(84)44(29-69)67-51(79)39(11-7-8-21-57)63-49(77)37(58)24-33-12-16-35(71)17-13-33/h4-6,9-10,12-19,31,37-45,69-72H,7-8,11,20-30,57-58H2,1-3H3,(H2,59,76)(H,60,78)(H,61,73)(H,62,80)(H,63,77)(H,64,81)(H,65,83)(H,66,84)(H,67,79)(H,68,82)(H,74,75)/t37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Marshall University School of Medicine and Huntington VA Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at bfSPR to produce increase in intracellular [Ca2+] |
J Med Chem 43: 1741-53 (2000)
BindingDB Entry DOI: 10.7270/Q27D2TCJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50140771

(((2R,3R)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES Ic1ccccc1CN[C@@H]1C2CCN(CC2)[C@@H]1C(c1ccccc1)c1ccccc1 |r,wU:9.9,16.19,TLB:17:16:12.11:14.15,THB:8:9:12.11:14.15,(23.35,-28.77,;24.59,-29.47,;25.82,-28.74,;27.08,-29.44,;27.09,-30.88,;25.86,-31.6,;24.61,-30.89,;23.38,-31.62,;23.39,-33.05,;22.16,-33.77,;20.81,-33.16,;19.33,-33.8,;19.53,-35.19,;21.07,-34.54,;21.32,-32.62,;20.87,-31.51,;22.44,-35.17,;23.21,-36.5,;24.75,-36.5,;25.52,-37.85,;27.07,-37.85,;27.84,-36.5,;27.07,-35.16,;25.52,-35.17,;22.43,-37.84,;20.88,-37.84,;20.11,-39.17,;20.88,-40.52,;22.44,-40.51,;23.21,-39.17,)| Show InChI InChI=1S/C27H29IN2/c28-24-14-8-7-13-23(24)19-29-26-22-15-17-30(18-16-22)27(26)25(20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-14,22,25-27,29H,15-19H2/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24.4 | n/a | n/a | n/a | n/a |
Tom's of Maine
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR140333 from human recombinant NK1 receptor expressed in CHO cells |
J Nat Prod 69: 432-5 (2006)
Article DOI: 10.1021/np058114h
BindingDB Entry DOI: 10.7270/Q2V98907 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030212

((S)-2-{2-[(2R,5S,8R)-5,8-Dibenzyl-2-(3-guanidino-p...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CN1CCCNC(=O)CCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C44H65N11O8S/c1-28(2)24-33(41(61)52-31(39(45)59)19-23-64-3)51-38(58)27-55-22-11-21-48-36(56)17-18-37(57)50-32(16-10-20-49-44(46)47)40(60)53-34(25-29-12-6-4-7-13-29)42(62)54-35(43(55)63)26-30-14-8-5-9-15-30/h4-9,12-15,28,31-35H,10-11,16-27H2,1-3H3,(H2,45,59)(H,48,56)(H,50,57)(H,51,58)(H,52,61)(H,53,60)(H,54,62)(H4,46,47,49)/t31-,32+,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Technische Universität München
Curated by ChEMBL
| Assay Description
Tested for the biological activity and selectivity against NK-1 receptor |
J Med Chem 37: 2145-52 (1994)
BindingDB Entry DOI: 10.7270/Q2NG4PNN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50559988
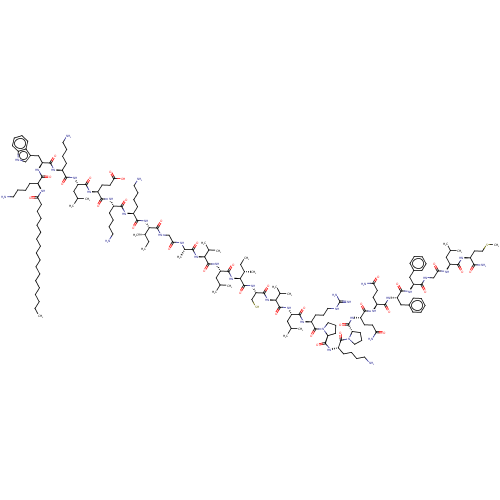
(CHEMBL4794099)Show SMILES CCCCCCCCCCCCCCCCCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NK1R (unknown origin) expressed in HEK293 cells assessed as reduction in cell viability incubated for 96 hrs by MTT assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127353
BindingDB Entry DOI: 10.7270/Q2SB49F8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50559987

(CHEMBL4750714)Show SMILES CCCCCCCCCCCCCCCCCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NK1R (unknown origin) expressed in HEK293 cells assessed as reduction in cell viability incubated for 96 hrs by MTT assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127353
BindingDB Entry DOI: 10.7270/Q2SB49F8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030214
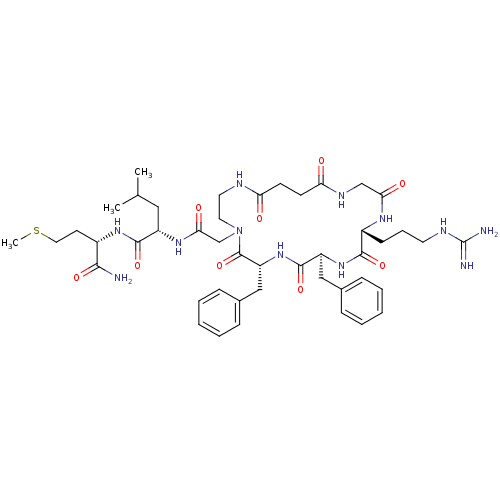
((S)-2-{2-[(6R,9S,12R)-6,9-Dibenzyl-12-(3-guanidino...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CN1CCNC(=O)CCC(=O)NCC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C45H66N12O9S/c1-28(2)23-33(42(64)54-31(40(46)62)18-22-67-3)53-39(61)27-57-21-20-49-36(58)16-17-37(59)51-26-38(60)52-32(15-10-19-50-45(47)48)41(63)55-34(24-29-11-6-4-7-12-29)43(65)56-35(44(57)66)25-30-13-8-5-9-14-30/h4-9,11-14,28,31-35H,10,15-27H2,1-3H3,(H2,46,62)(H,49,58)(H,51,59)(H,52,60)(H,53,61)(H,54,64)(H,55,63)(H,56,65)(H4,47,48,50)/t31-,32+,33-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Technische Universität München
Curated by ChEMBL
| Assay Description
Tested for the biological activity and selectivity against NK-1 receptor |
J Med Chem 37: 2145-52 (1994)
BindingDB Entry DOI: 10.7270/Q2NG4PNN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50261729
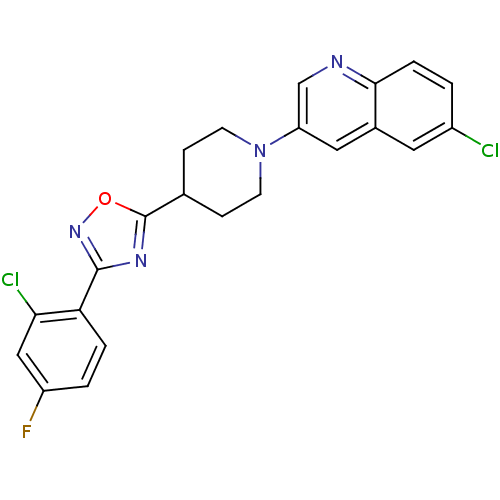
(6-chloro-3-(4-(3-(2-chloro-4-fluorophenyl)-1,2,4-o...)Show SMILES Fc1ccc(-c2noc(n2)C2CCN(CC2)c2cnc3ccc(Cl)cc3c2)c(Cl)c1 Show InChI InChI=1S/C22H17Cl2FN4O/c23-15-1-4-20-14(9-15)10-17(12-26-20)29-7-5-13(6-8-29)22-27-21(28-30-22)18-3-2-16(25)11-19(18)24/h1-4,9-13H,5-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at NK1 receptor (unknown origin) |
Bioorg Med Chem Lett 18: 4267-74 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.096
BindingDB Entry DOI: 10.7270/Q21C1WQ8 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50456633

(CHEMBL4215185)Show SMILES Cc1ccc(cc1)[S+]([O-])C1=C(OC(NC(=O)C(F)(F)F)=C(C#N)[C@H]1c1ccc(cc1)[N+]([O-])=O)c1ccccn1 |r,t:10,20| Show InChI InChI=1S/C26H17F3N4O5S/c1-15-5-11-18(12-6-15)39(37)23-21(16-7-9-17(10-8-16)33(35)36)19(14-30)24(32-25(34)26(27,28)29)38-22(23)20-4-2-3-13-31-20/h2-13,21H,1H3,(H,32,34)/t21-,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Partial agonist activity at His6-tagged NK1 receptor (unknown origin) expressed in CHO cells assessed as IP1 accumulation measured after 30 mins by H... |
Eur J Med Chem 138: 644-660 (2017)
Article DOI: 10.1016/j.ejmech.2017.06.056
BindingDB Entry DOI: 10.7270/Q2930WSH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















