 Found 241 hits of ec50 data for polymerid = 2125,50000659
Found 241 hits of ec50 data for polymerid = 2125,50000659 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329178
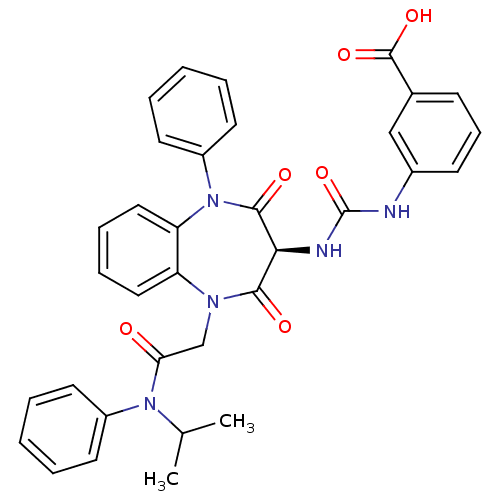
((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)[C@@H](NC(=O)Nc2cccc(c2)C(O)=O)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H31N5O6/c1-22(2)38(25-14-5-3-6-15-25)29(40)21-37-27-18-9-10-19-28(27)39(26-16-7-4-8-17-26)32(42)30(31(37)41)36-34(45)35-24-13-11-12-23(20-24)33(43)44/h3-20,22,30H,21H2,1-2H3,(H,43,44)(H2,35,36,45)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.316 nM (Rvb = 7 to 14... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329178

((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)[C@@H](NC(=O)Nc2cccc(c2)C(O)=O)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H31N5O6/c1-22(2)38(25-14-5-3-6-15-25)29(40)21-37-27-18-9-10-19-28(27)39(26-16-7-4-8-17-26)32(42)30(31(37)41)36-34(45)35-24-13-11-12-23(20-24)33(43)44/h3-20,22,30H,21H2,1-2H3,(H,43,44)(H2,35,36,45)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.1 nM (Rvb = 7 to 14 p... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329178

((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)[C@@H](NC(=O)Nc2cccc(c2)C(O)=O)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H31N5O6/c1-22(2)38(25-14-5-3-6-15-25)29(40)21-37-27-18-9-10-19-28(27)39(26-16-7-4-8-17-26)32(42)30(31(37)41)36-34(45)35-24-13-11-12-23(20-24)33(43)44/h3-20,22,30H,21H2,1-2H3,(H,43,44)(H2,35,36,45)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.01 nM (Rvb = 7 to 14 ... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832
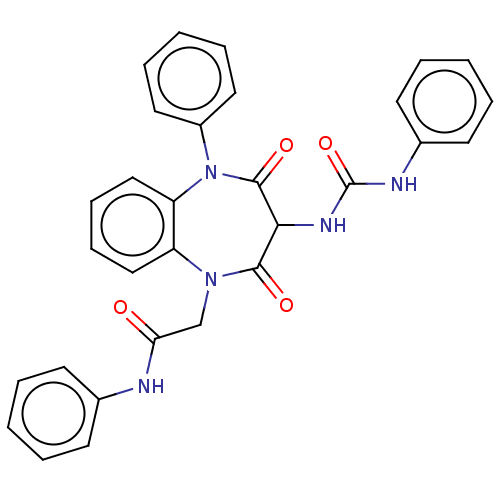
(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832

(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419
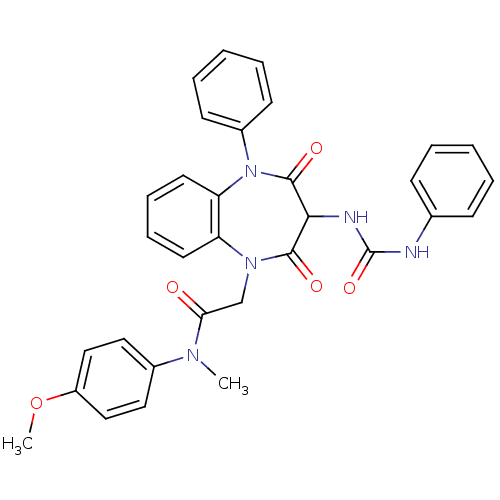
(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50061829
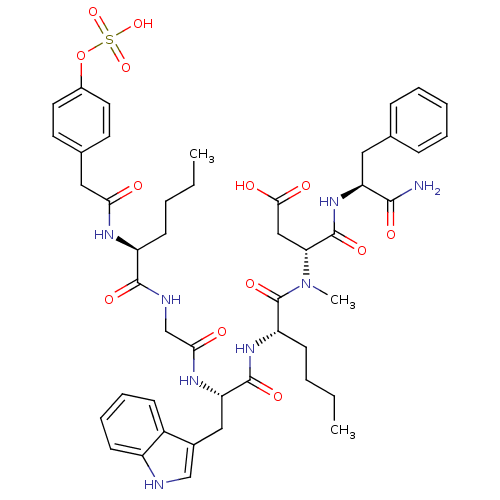
((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-({(S)-2-[...)Show SMILES CCCC[C@H](NC(=O)Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H60N8O13S/c1-4-6-16-35(51-40(56)24-30-19-21-32(22-20-30)68-69(65,66)67)44(61)50-28-41(57)52-38(25-31-27-49-34-18-12-11-15-33(31)34)45(62)53-36(17-7-5-2)47(64)55(3)39(26-42(58)59)46(63)54-37(43(48)60)23-29-13-9-8-10-14-29/h8-15,18-22,27,35-39,49H,4-7,16-17,23-26,28H2,1-3H3,(H2,48,60)(H,50,61)(H,51,56)(H,52,57)(H,53,62)(H,54,63)(H,58,59)(H,65,66,67)/t35-,36-,37-,38-,39+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245189

(3-((R)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36FN5O5/c1-4-48-29-10-7-9-26(18-29)43-22-34(40-35(43)31-13-12-23(2)16-33(31)38)36(45)42-15-14-41(21-28(42)20-39-24(3)44)27-17-25-8-5-6-11-30(25)32(19-27)37(46)47/h5-13,16-19,22,28H,4,14-15,20-21H2,1-3H3,(H,39,44)(H,46,47)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245188
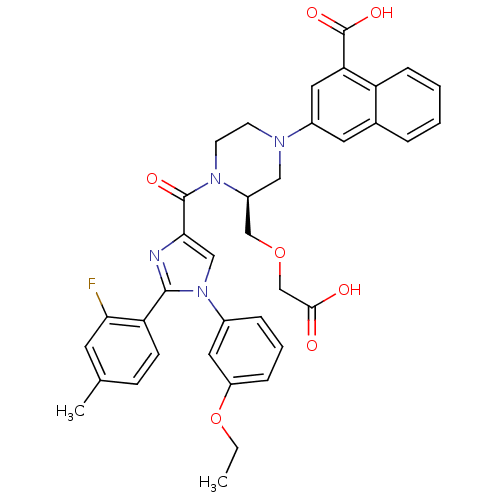
(3-((S)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H35FN4O7/c1-3-49-28-9-6-8-25(17-28)42-20-33(39-35(42)30-12-11-23(2)15-32(30)38)36(45)41-14-13-40(19-27(41)21-48-22-34(43)44)26-16-24-7-4-5-10-29(24)31(18-26)37(46)47/h4-12,15-18,20,27H,3,13-14,19,21-22H2,1-2H3,(H,43,44)(H,46,47)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50245192
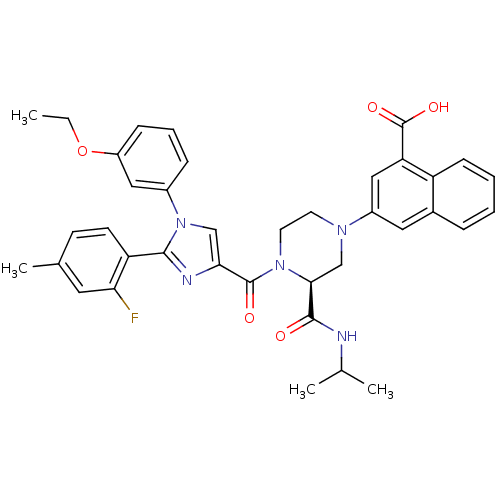
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832

(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1000 nM (Rvb = 7 to 14 ... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245182

(3-((S)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37N5O5/c1-4-47-31-10-7-9-28(19-31)42-23-34(39-35(42)26-14-12-24(2)13-15-26)36(44)41-17-16-40(22-30(41)21-38-25(3)43)29-18-27-8-5-6-11-32(27)33(20-29)37(45)46/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,38,43)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0437 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263226
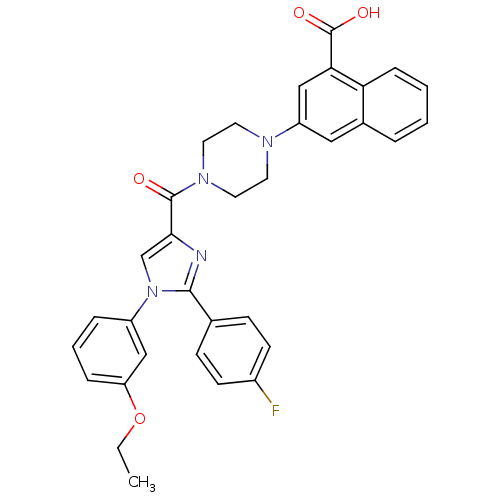
(3-(4-(1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imi...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H29FN4O4/c1-2-42-27-8-5-7-25(19-27)38-21-30(35-31(38)22-10-12-24(34)13-11-22)32(39)37-16-14-36(15-17-37)26-18-23-6-3-4-9-28(23)29(20-26)33(40)41/h3-13,18-21H,2,14-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263227

(3-(4-(2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H28F2N4O4/c1-2-43-25-8-5-7-23(18-25)39-20-30(36-31(39)27-11-10-22(34)17-29(27)35)32(40)38-14-12-37(13-15-38)24-16-21-6-3-4-9-26(21)28(19-24)33(41)42/h3-11,16-20H,2,12-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245186
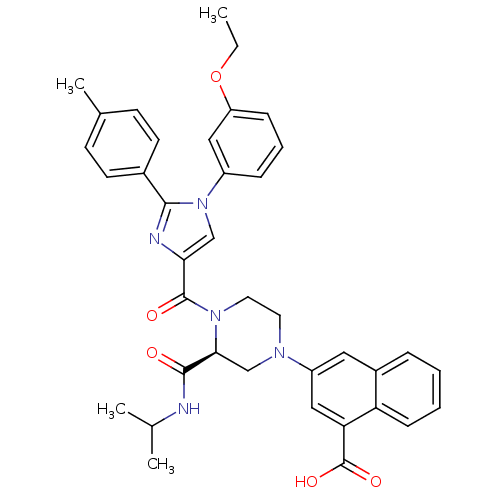
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517380

(CHEMBL4464703)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](CCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)NC(=O)Cc1ccc(O)cc1)C(=O)N(C)[C@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C82H122N12O22/c1-4-5-29-64(80(109)94(3)68(52-76(104)105)81(110)93(2)67(77(83)106)49-57-26-19-18-20-27-57)92-79(108)66(51-59-53-87-62-30-24-23-28-61(59)62)91-72(99)54-88-78(107)63(89-71(98)50-58-34-36-60(95)37-35-58)31-25-40-84-73(100)55-115-47-46-114-44-42-86-74(101)56-116-48-45-113-43-41-85-69(96)39-38-65(82(111)112)90-70(97)32-21-16-14-12-10-8-6-7-9-11-13-15-17-22-33-75(102)103/h18-20,23-24,26-28,30,34-37,53,63-68,87,95H,4-17,21-22,25,29,31-33,38-52,54-56H2,1-3H3,(H2,83,106)(H,84,100)(H,85,96)(H,86,101)(H,88,107)(H,89,98)(H,90,97)(H,91,99)(H,92,108)(H,102,103)(H,104,105)(H,111,112)/t63-,64+,65+,66+,67+,68-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0603 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343721
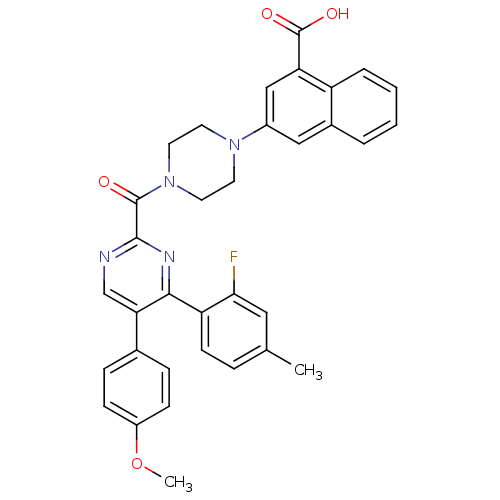
(3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...)Show SMILES COc1ccc(cc1)-c1cnc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H29FN4O4/c1-21-7-12-27(30(35)17-21)31-29(22-8-10-25(43-2)11-9-22)20-36-32(37-31)33(40)39-15-13-38(14-16-39)24-18-23-5-3-4-6-26(23)28(19-24)34(41)42/h3-12,17-20H,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343722
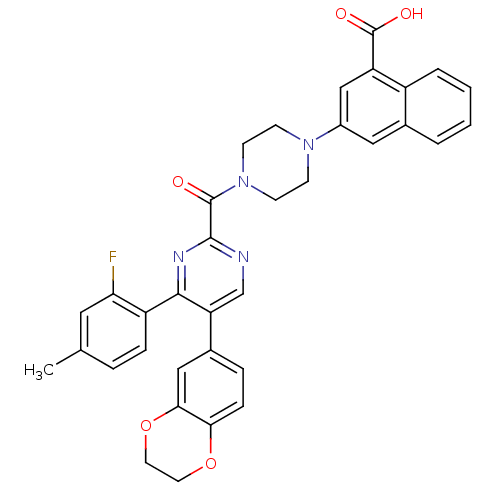
(3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...)Show SMILES Cc1ccc(c(F)c1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C35H29FN4O5/c1-21-6-8-26(29(36)16-21)32-28(23-7-9-30-31(18-23)45-15-14-44-30)20-37-33(38-32)34(41)40-12-10-39(11-13-40)24-17-22-4-2-3-5-25(22)27(19-24)35(42)43/h2-9,16-20H,10-15H2,1H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m... |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245201
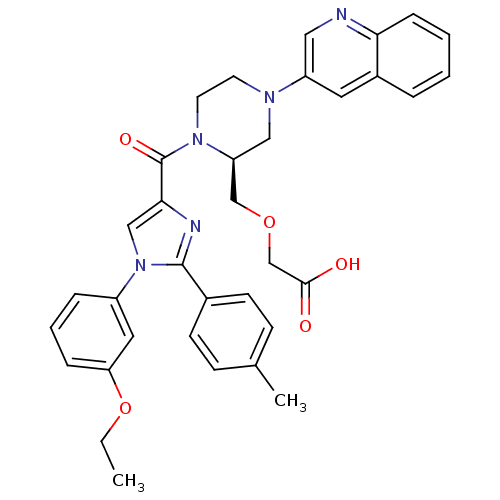
(3-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517379
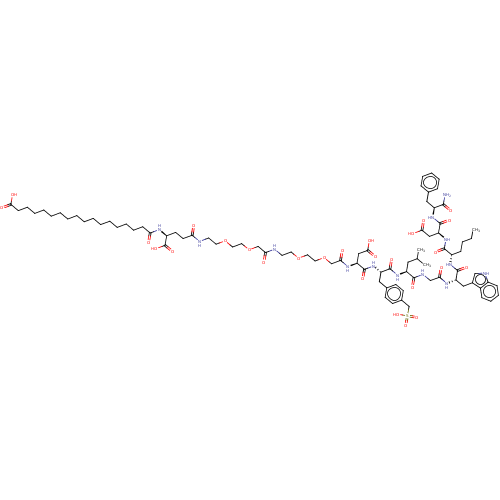
(CHEMBL4453982)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(CS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O27S/c1-4-5-28-64(82(114)100-71(51-79(110)111)86(118)97-66(80(88)112)47-58-25-19-18-20-26-58)96-84(116)69(49-61-52-91-63-29-24-23-27-62(61)63)94-74(103)53-92-81(113)67(46-57(2)3)98-83(115)68(48-59-32-34-60(35-33-59)56-128(121,122)123)99-85(117)70(50-78(108)109)95-76(105)55-127-45-43-125-41-39-90-75(104)54-126-44-42-124-40-38-89-72(101)37-36-65(87(119)120)93-73(102)30-21-16-14-12-10-8-6-7-9-11-13-15-17-22-31-77(106)107/h18-20,23-27,29,32-35,52,57,64-71,91H,4-17,21-22,28,30-31,36-51,53-56H2,1-3H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,116)(H,97,118)(H,98,115)(H,99,117)(H,100,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0676 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245192

(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245191
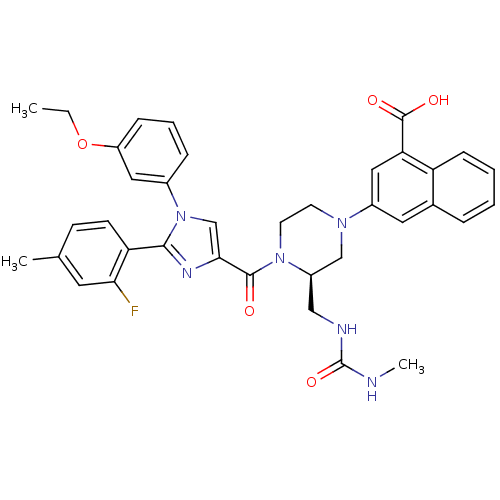
(3-((R)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37FN6O5/c1-4-49-28-10-7-9-25(18-28)44-22-33(41-34(44)30-13-12-23(2)16-32(30)38)35(45)43-15-14-42(21-27(43)20-40-37(48)39-3)26-17-24-8-5-6-11-29(24)31(19-26)36(46)47/h5-13,16-19,22,27H,4,14-15,20-21H2,1-3H3,(H,46,47)(H2,39,40,48)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263230
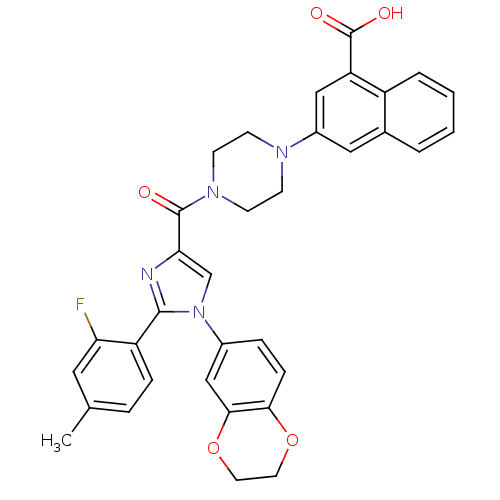
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2...)Show SMILES Cc1ccc(-c2nc(cn2-c2ccc3OCCOc3c2)C(=O)N2CCN(CC2)c2cc(C(O)=O)c3ccccc3c2)c(F)c1 Show InChI InChI=1S/C34H29FN4O5/c1-21-6-8-26(28(35)16-21)32-36-29(20-39(32)23-7-9-30-31(19-23)44-15-14-43-30)33(40)38-12-10-37(11-13-38)24-17-22-4-2-3-5-25(22)27(18-24)34(41)42/h2-9,16-20H,10-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262862

(3-(4-(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazole-4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H30N4O4/c1-22-10-12-23(13-11-22)31-34-30(21-37(31)25-7-5-8-27(19-25)41-2)32(38)36-16-14-35(15-17-36)26-18-24-6-3-4-9-28(24)29(20-26)33(39)40/h3-13,18-21H,14-17H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245185

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245205
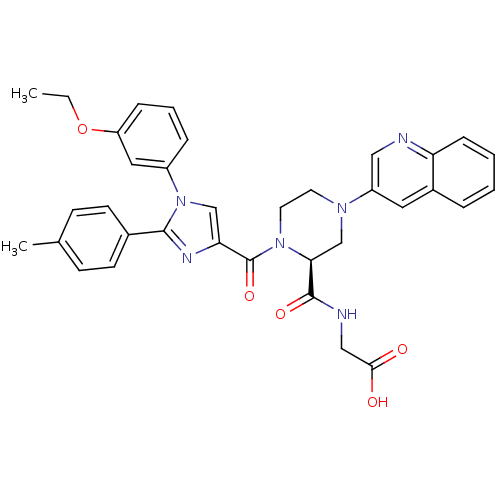
(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263229
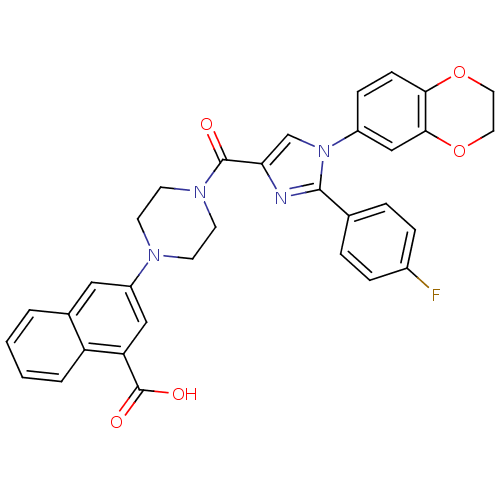
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4...)Show SMILES OC(=O)c1cc(cc2ccccc12)N1CCN(CC1)C(=O)c1cn(c(n1)-c1ccc(F)cc1)-c1ccc2OCCOc2c1 Show InChI InChI=1S/C33H27FN4O5/c34-23-7-5-21(6-8-23)31-35-28(20-38(31)24-9-10-29-30(19-24)43-16-15-42-29)32(39)37-13-11-36(12-14-37)25-17-22-3-1-2-4-26(22)27(18-25)33(40)41/h1-10,17-20H,11-16H2,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180
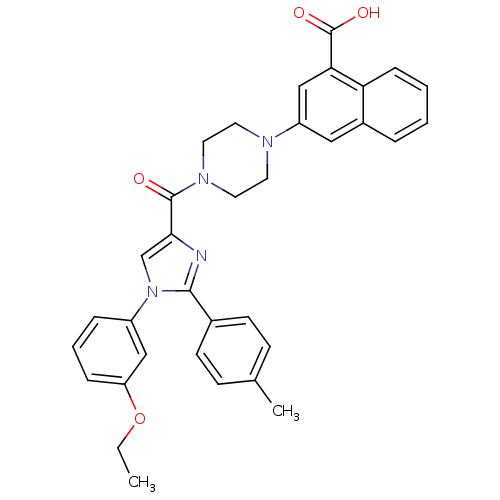
(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180

(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245183
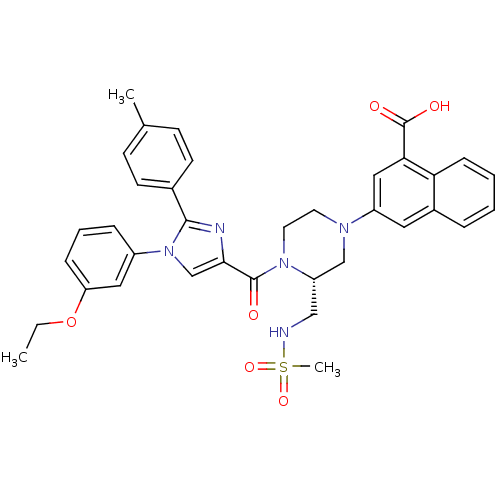
(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H37N5O6S/c1-4-47-30-10-7-9-27(19-30)41-23-33(38-34(41)25-14-12-24(2)13-15-25)35(42)40-17-16-39(22-29(40)21-37-48(3,45)46)28-18-26-8-5-6-11-31(26)32(20-28)36(43)44/h5-15,18-20,23,29,37H,4,16-17,21-22H2,1-3H3,(H,43,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
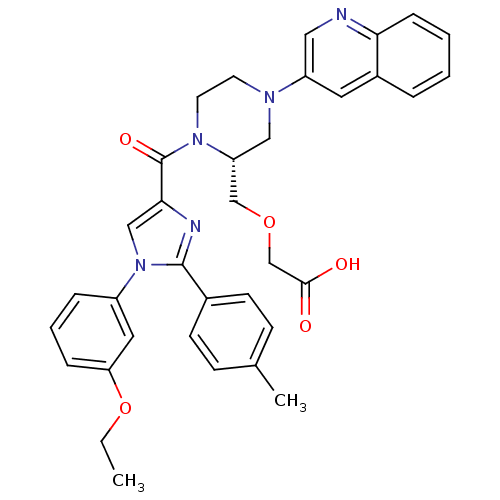
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245195
(2-(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
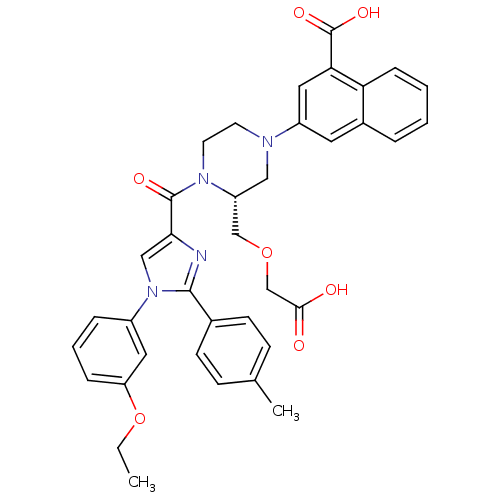
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245181
(3-((R)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36N4O7/c1-3-48-30-9-6-8-27(18-30)41-21-33(38-35(41)25-13-11-24(2)12-14-25)36(44)40-16-15-39(20-29(40)22-47-23-34(42)43)28-17-26-7-4-5-10-31(26)32(19-28)37(45)46/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,42,43)(H,45,46)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245202
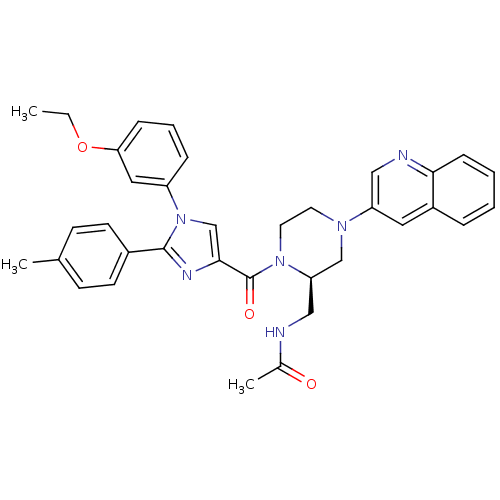
(CHEMBL503331 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245190
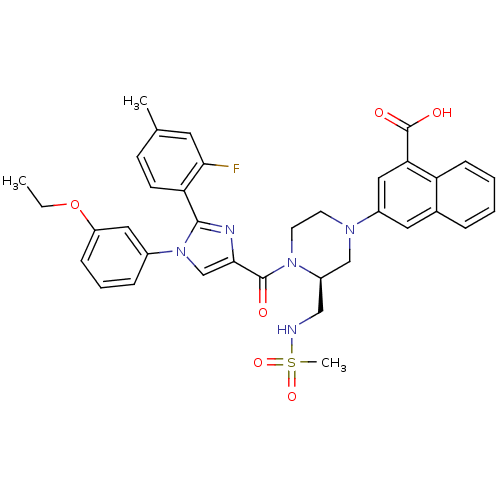
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H36FN5O6S/c1-4-48-28-10-7-9-25(18-28)42-22-33(39-34(42)30-13-12-23(2)16-32(30)37)35(43)41-15-14-40(21-27(41)20-38-49(3,46)47)26-17-24-8-5-6-11-29(24)31(19-26)36(44)45/h5-13,16-19,22,27,38H,4,14-15,20-21H2,1-3H3,(H,44,45)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245196
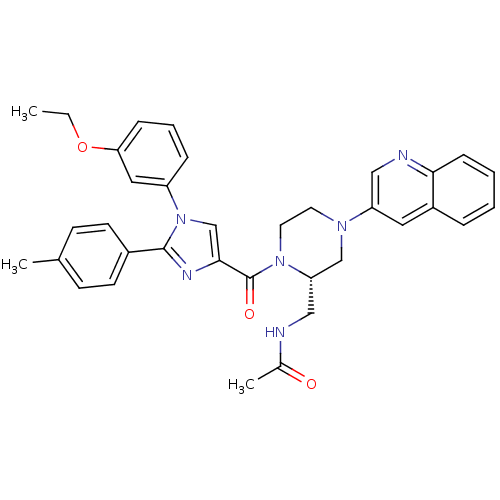
(CHEMBL450443 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517371
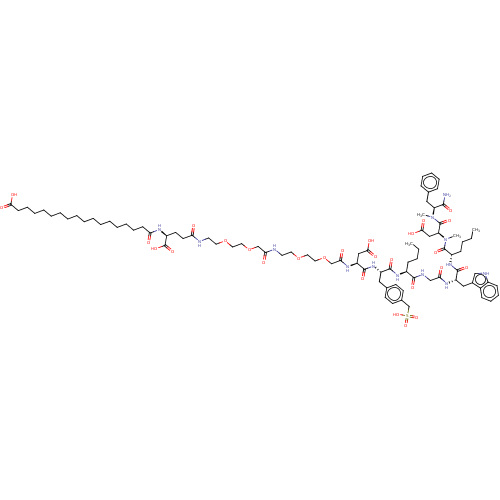
(CHEMBL4475304)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(CS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C89H133N13O27S/c1-5-7-31-66(83(115)94-56-76(105)96-70(52-63-55-93-65-33-27-26-30-64(63)65)85(117)99-67(32-8-6-2)87(119)102(4)73(54-81(112)113)88(120)101(3)72(82(90)114)51-60-28-22-21-23-29-60)98-84(116)69(50-61-36-38-62(39-37-61)59-130(123,124)125)100-86(118)71(53-80(110)111)97-78(107)58-129-49-47-127-45-43-92-77(106)57-128-48-46-126-44-42-91-74(103)41-40-68(89(121)122)95-75(104)34-24-19-17-15-13-11-9-10-12-14-16-18-20-25-35-79(108)109/h21-23,26-30,33,36-39,55,66-73,93H,5-20,24-25,31-32,34-35,40-54,56-59H2,1-4H3,(H2,90,114)(H,91,103)(H,92,106)(H,94,115)(H,95,104)(H,96,105)(H,97,107)(H,98,116)(H,99,117)(H,100,118)(H,108,109)(H,110,111)(H,112,113)(H,121,122)(H,123,124,125)/t66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517355
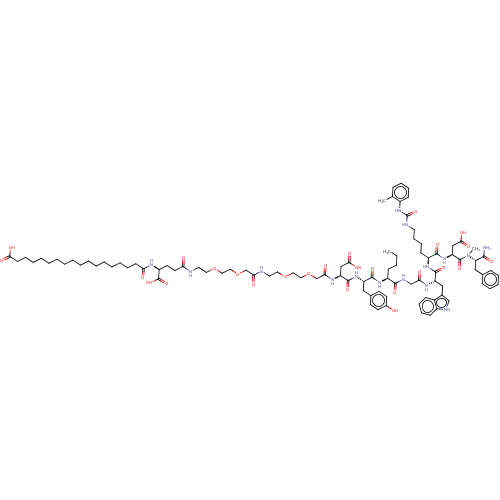
(CHEMBL4471525)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C95H137N15O26/c1-4-5-33-71(88(124)101-60-81(114)103-75(56-66-59-100-70-35-26-24-32-68(66)70)91(127)106-72(36-27-28-45-99-95(132)109-69-34-25-23-29-63(69)2)89(125)108-77(58-86(121)122)93(129)110(3)78(87(96)123)55-64-30-19-18-20-31-64)105-90(126)74(54-65-39-41-67(111)42-40-65)107-92(128)76(57-85(119)120)104-83(116)62-136-53-51-134-49-47-98-82(115)61-135-52-50-133-48-46-97-79(112)44-43-73(94(130)131)102-80(113)37-21-16-14-12-10-8-6-7-9-11-13-15-17-22-38-84(117)118/h18-20,23-26,29-32,34-35,39-42,59,71-78,100,111H,4-17,21-22,27-28,33,36-38,43-58,60-62H2,1-3H3,(H2,96,123)(H,97,112)(H,98,115)(H,101,124)(H,102,113)(H,103,114)(H,104,116)(H,105,126)(H,106,127)(H,107,128)(H,108,125)(H,117,118)(H,119,120)(H,121,122)(H,130,131)(H2,99,109,132)/t71-,72-,73-,74-,75-,76-,77-,78-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517331
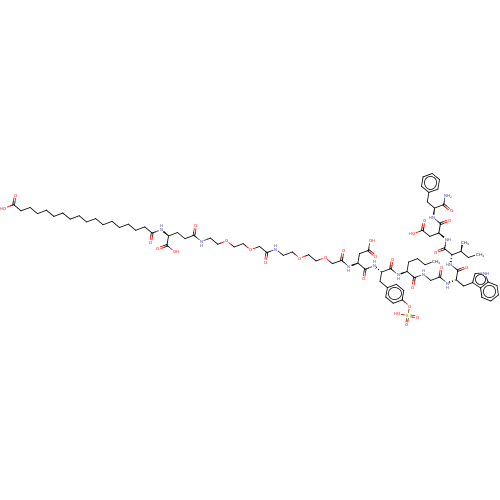
(CHEMBL4467338)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H127N13O28S/c1-4-6-29-63(80(112)91-53-72(102)93-67(49-59-52-90-62-30-25-24-28-61(59)62)84(116)99-78(56(3)5-2)85(117)98-69(51-77(109)110)83(115)96-65(79(87)111)47-57-26-20-19-21-27-57)95-81(113)66(48-58-33-35-60(36-34-58)127-128(120,121)122)97-82(114)68(50-76(107)108)94-74(104)55-126-46-44-124-42-40-89-73(103)54-125-45-43-123-41-39-88-70(100)38-37-64(86(118)119)92-71(101)31-22-17-15-13-11-9-7-8-10-12-14-16-18-23-32-75(105)106/h19-21,24-28,30,33-36,52,56,63-69,78,90H,4-18,22-23,29,31-32,37-51,53-55H2,1-3H3,(H2,87,111)(H,88,100)(H,89,103)(H,91,112)(H,92,101)(H,93,102)(H,94,104)(H,95,113)(H,96,115)(H,97,114)(H,98,117)(H,99,116)(H,105,106)(H,107,108)(H,109,110)(H,118,119)(H,120,121,122)/t56-,63-,64-,65-,66-,67-,68-,69-,78-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50245186

(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50245205

(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517339
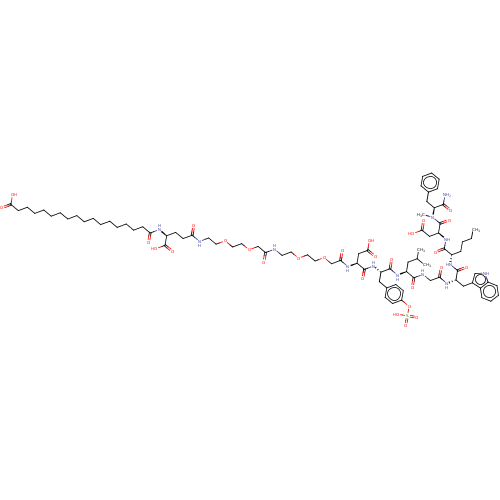
(CHEMBL4551834)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O28S/c1-5-6-29-64(82(114)99-70(52-79(110)111)86(118)100(4)71(80(88)112)49-58-26-20-19-21-27-58)96-84(116)68(50-60-53-91-63-30-25-24-28-62(60)63)94-74(103)54-92-81(113)66(47-57(2)3)97-83(115)67(48-59-33-35-61(36-34-59)128-129(121,122)123)98-85(117)69(51-78(108)109)95-76(105)56-127-46-44-125-42-40-90-75(104)55-126-45-43-124-41-39-89-72(101)38-37-65(87(119)120)93-73(102)31-22-17-15-13-11-9-7-8-10-12-14-16-18-23-32-77(106)107/h19-21,24-28,30,33-36,53,57,64-71,91H,5-18,22-23,29,31-32,37-52,54-56H2,1-4H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,116)(H,97,115)(H,98,117)(H,99,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50517356

(CHEMBL4557012)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1C)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C96H139N15O29S/c1-5-6-34-72(89(124)102-61-82(114)104-76(57-67-60-101-71-36-27-25-33-69(67)71)91(126)107-73(37-28-29-46-100-96(132)109-70-35-26-24-30-64(70)2)93(128)111(4)79(59-87(121)122)94(129)110(3)78(88(97)123)56-65-31-20-19-21-32-65)106-90(125)75(55-66-40-42-68(43-41-66)140-141(133,134)135)108-92(127)77(58-86(119)120)105-84(116)63-139-54-52-137-50-48-99-83(115)62-138-53-51-136-49-47-98-80(112)45-44-74(95(130)131)103-81(113)38-22-17-15-13-11-9-7-8-10-12-14-16-18-23-39-85(117)118/h19-21,24-27,30-33,35-36,40-43,60,72-79,101H,5-18,22-23,28-29,34,37-39,44-59,61-63H2,1-4H3,(H2,97,123)(H,98,112)(H,99,115)(H,102,124)(H,103,113)(H,104,114)(H,105,116)(H,106,125)(H,107,126)(H,108,127)(H,117,118)(H,119,120)(H,121,122)(H,130,131)(H2,100,109,132)(H,133,134,135)/t72-,73-,74-,75-,76-,77-,78-,79-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263186
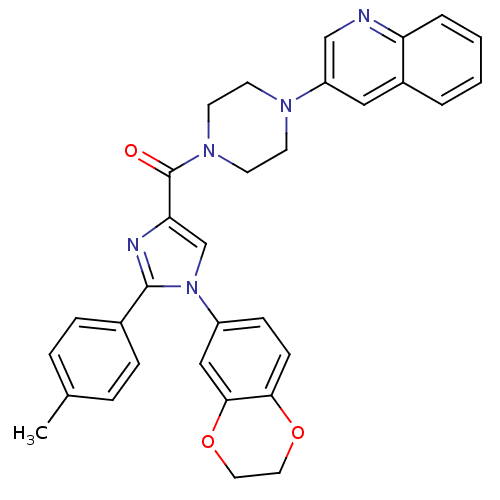
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H29N5O3/c1-22-6-8-23(9-7-22)31-34-28(21-37(31)25-10-11-29-30(19-25)40-17-16-39-29)32(38)36-14-12-35(13-15-36)26-18-24-4-2-3-5-27(24)33-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity against human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50343722

(3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...)Show SMILES Cc1ccc(c(F)c1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C35H29FN4O5/c1-21-6-8-26(29(36)16-21)32-28(23-7-9-30-31(18-23)45-15-14-44-30)20-37-33(38-32)34(41)40-12-10-39(11-13-40)24-17-22-4-2-3-5-25(22)27(19-24)35(42)43/h2-9,16-20H,10-15H2,1H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse CCK1 receptor expressed in CHO Flip cells assessed as increase in radio labeled inositol phosphate accumulation by Wallac m... |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1000 nM (Rvb = 7 to 14 ... |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728
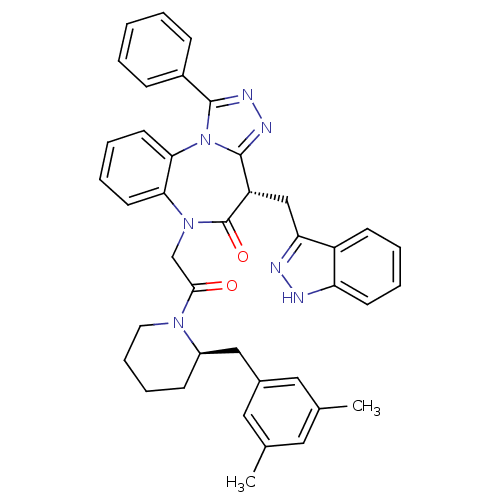
(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R T3.28V, T3.29S mutant expressed in CHO cells assessed as intracellular calcium response by fluorescence analysis |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data