 Found 123 hits of ec50 data for polymerid = 2126
Found 123 hits of ec50 data for polymerid = 2126 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Tom's of Maine
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8 from CCK2 receptor in human FGS7 Jurkat cells |
J Nat Prod 69: 432-5 (2006)
Article DOI: 10.1021/np058114h
BindingDB Entry DOI: 10.7270/Q2V98907 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517367
(CHEMBL4468861)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C85H123N13O30S/c1-2-3-26-61(80(114)98-68(50-77(110)111)84(118)96-64(78(86)112)46-55-23-17-16-18-24-55)94-82(116)66(48-57-51-89-60-27-22-21-25-59(57)60)92-71(101)52-90-79(113)62(35-37-75(106)107)95-81(115)65(47-56-30-32-58(33-31-56)128-129(121,122)123)97-83(117)67(49-76(108)109)93-73(103)54-127-45-43-125-41-39-88-72(102)53-126-44-42-124-40-38-87-69(99)36-34-63(85(119)120)91-70(100)28-19-14-12-10-8-6-4-5-7-9-11-13-15-20-29-74(104)105/h16-18,21-25,27,30-33,51,61-68,89H,2-15,19-20,26,28-29,34-50,52-54H2,1H3,(H2,86,112)(H,87,99)(H,88,102)(H,90,113)(H,91,100)(H,92,101)(H,93,103)(H,94,116)(H,95,115)(H,96,118)(H,97,117)(H,98,114)(H,104,105)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t61-,62-,63-,64-,65-,66-,67-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.295 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517352

(CHEMBL4570130)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O28S/c1-4-5-28-64(82(114)100-71(53-79(110)111)86(118)97-66(80(88)112)49-58-25-19-18-20-26-58)96-84(116)69(51-60-54-92-63-29-24-23-27-62(60)63)94-74(103)38-39-91-81(113)67(48-57(2)3)98-83(115)68(50-59-32-34-61(35-33-59)128-129(121,122)123)99-85(117)70(52-78(108)109)95-76(105)56-127-47-45-125-43-41-90-75(104)55-126-46-44-124-42-40-89-72(101)37-36-65(87(119)120)93-73(102)30-21-16-14-12-10-8-6-7-9-11-13-15-17-22-31-77(106)107/h18-20,23-27,29,32-35,54,57,64-71,92H,4-17,21-22,28,30-31,36-53,55-56H2,1-3H3,(H2,88,112)(H,89,101)(H,90,104)(H,91,113)(H,93,102)(H,94,103)(H,95,105)(H,96,116)(H,97,118)(H,98,115)(H,99,117)(H,100,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517332
(CHEMBL4522865)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C86H127N13O29S/c1-3-5-24-63(80(113)91-53-73(103)93-68(49-58-52-90-62-26-22-21-23-61(58)62)83(116)96-64(25-6-4-2)81(114)99-70(51-78(110)111)85(118)97-66(79(87)112)47-56-29-33-59(100)34-30-56)95-82(115)67(48-57-31-35-60(36-32-57)128-129(121,122)123)98-84(117)69(50-77(108)109)94-75(105)55-127-46-44-125-42-40-89-74(104)54-126-45-43-124-41-39-88-71(101)38-37-65(86(119)120)92-72(102)27-19-17-15-13-11-9-7-8-10-12-14-16-18-20-28-76(106)107/h21-23,26,29-36,52,63-70,90,100H,3-20,24-25,27-28,37-51,53-55H2,1-2H3,(H2,87,112)(H,88,101)(H,89,104)(H,91,113)(H,92,102)(H,93,103)(H,94,105)(H,95,115)(H,96,116)(H,97,118)(H,98,117)(H,99,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.324 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517335

(CHEMBL4547294)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OP(O)(O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H128N13O28P/c1-3-5-29-63(80(112)91-54-73(102)93-68(50-59-53-90-62-31-25-24-28-61(59)62)83(115)96-64(30-6-4-2)81(113)99-70(52-78(109)110)85(117)97-66(79(87)111)48-57-26-20-19-21-27-57)95-82(114)67(49-58-34-36-60(37-35-58)127-128(120,121)122)98-84(116)69(51-77(107)108)94-75(104)56-126-47-45-124-43-41-89-74(103)55-125-46-44-123-42-40-88-71(100)39-38-65(86(118)119)92-72(101)32-22-17-15-13-11-9-7-8-10-12-14-16-18-23-33-76(105)106/h19-21,24-28,31,34-37,53,63-70,90H,3-18,22-23,29-30,32-33,38-52,54-56H2,1-2H3,(H2,87,111)(H,88,100)(H,89,103)(H,91,112)(H,92,101)(H,93,102)(H,94,104)(H,95,114)(H,96,115)(H,97,117)(H,98,116)(H,99,113)(H,105,106)(H,107,108)(H,109,110)(H,118,119)(H2,120,121,122)/t63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517351

(CHEMBL4516868)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C89H131N13O28S/c1-3-5-30-65(82(114)100-72(55-80(111)112)86(118)98-68(81(90)113)51-59-27-20-19-21-28-59)96-84(116)70(53-61-56-93-64-32-25-24-29-63(61)64)101-87(119)73-33-26-44-102(73)88(120)66(31-6-4-2)97-83(115)69(52-60-36-38-62(39-37-60)130-131(123,124)125)99-85(117)71(54-79(109)110)95-77(106)58-129-50-48-127-46-43-92-76(105)57-128-49-47-126-45-42-91-74(103)41-40-67(89(121)122)94-75(104)34-22-17-15-13-11-9-7-8-10-12-14-16-18-23-35-78(107)108/h19-21,24-25,27-29,32,36-39,56,65-73,93H,3-18,22-23,26,30-31,33-35,40-55,57-58H2,1-2H3,(H2,90,113)(H,91,103)(H,92,105)(H,94,104)(H,95,106)(H,96,116)(H,97,115)(H,98,118)(H,99,117)(H,100,114)(H,101,119)(H,107,108)(H,109,110)(H,111,112)(H,121,122)(H,123,124,125)/t65-,66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517347

(CHEMBL4555341)Show SMILES CCCC[C@H](NC(=O)[C@H](CNC(=O)CCCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C85H130N14O27/c1-3-5-30-60(79(115)91-53-71(103)93-64(48-57-51-89-59-32-25-24-29-58(57)59)81(117)96-61(31-6-4-2)80(116)98-66(50-77(112)113)83(119)97-63(78(86)114)47-56-27-20-19-21-28-56)95-84(120)67(52-90-68(100)34-26-36-75(108)109)99-82(118)65(49-76(110)111)94-73(105)55-126-46-44-124-42-40-88-72(104)54-125-45-43-123-41-39-87-69(101)38-37-62(85(121)122)92-70(102)33-22-17-15-13-11-9-7-8-10-12-14-16-18-23-35-74(106)107/h19-21,24-25,27-29,32,51,60-67,89H,3-18,22-23,26,30-31,33-50,52-55H2,1-2H3,(H2,86,114)(H,87,101)(H,88,104)(H,90,100)(H,91,115)(H,92,102)(H,93,103)(H,94,105)(H,95,120)(H,96,117)(H,97,119)(H,98,116)(H,99,118)(H,106,107)(H,108,109)(H,110,111)(H,112,113)(H,121,122)/t60-,61-,62-,63-,64-,65-,66-,67-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517382
(CHEMBL4563364)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C90H129N13O28S/c1-3-5-31-67(84(116)95-56-77(106)97-72(52-62-55-94-66-33-24-23-30-65(62)66)87(119)100-68(32-6-4-2)85(117)103-74(54-82(113)114)89(121)101-70(83(91)115)51-61-28-25-27-60-26-21-22-29-64(60)61)99-86(118)71(50-59-36-38-63(39-37-59)131-132(124,125)126)102-88(120)73(53-81(111)112)98-79(108)58-130-49-47-128-45-43-93-78(107)57-129-48-46-127-44-42-92-75(104)41-40-69(90(122)123)96-76(105)34-19-17-15-13-11-9-7-8-10-12-14-16-18-20-35-80(109)110/h21-30,33,36-39,55,67-74,94H,3-20,31-32,34-35,40-54,56-58H2,1-2H3,(H2,91,115)(H,92,104)(H,93,107)(H,95,116)(H,96,105)(H,97,106)(H,98,108)(H,99,118)(H,100,119)(H,101,121)(H,102,120)(H,103,117)(H,109,110)(H,111,112)(H,113,114)(H,122,123)(H,124,125,126)/t67-,68-,69-,70-,71-,72-,73-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517336
(CHEMBL4459331)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C85H124N14O29S/c1-2-3-26-61(79(113)91-52-72(103)93-66(48-57-51-90-60-27-22-21-25-59(57)60)82(116)96-62(34-36-69(86)100)80(114)99-68(50-77(110)111)84(118)97-64(78(87)112)46-55-23-17-16-18-24-55)95-81(115)65(47-56-30-32-58(33-31-56)128-129(121,122)123)98-83(117)67(49-76(108)109)94-74(105)54-127-45-43-125-41-39-89-73(104)53-126-44-42-124-40-38-88-70(101)37-35-63(85(119)120)92-71(102)28-19-14-12-10-8-6-4-5-7-9-11-13-15-20-29-75(106)107/h16-18,21-25,27,30-33,51,61-68,90H,2-15,19-20,26,28-29,34-50,52-54H2,1H3,(H2,86,100)(H2,87,112)(H,88,101)(H,89,104)(H,91,113)(H,92,102)(H,93,103)(H,94,105)(H,95,115)(H,96,116)(H,97,118)(H,98,117)(H,99,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t61-,62-,63-,64-,65-,66-,67-,68-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.417 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517359
(CHEMBL4534236)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C88H128N14O28S/c1-3-5-27-65(82(115)94-54-75(105)96-70(49-59-53-93-64-30-24-22-26-62(59)64)85(118)99-66(28-6-4-2)83(116)102-72(51-80(112)113)87(120)100-68(81(89)114)48-58-52-92-63-29-23-21-25-61(58)63)98-84(117)69(47-57-33-35-60(36-34-57)130-131(123,124)125)101-86(119)71(50-79(110)111)97-77(107)56-129-46-44-127-42-40-91-76(106)55-128-45-43-126-41-39-90-73(103)38-37-67(88(121)122)95-74(104)31-19-17-15-13-11-9-7-8-10-12-14-16-18-20-32-78(108)109/h21-26,29-30,33-36,52-53,65-72,92-93H,3-20,27-28,31-32,37-51,54-56H2,1-2H3,(H2,89,114)(H,90,103)(H,91,106)(H,94,115)(H,95,104)(H,96,105)(H,97,107)(H,98,117)(H,99,118)(H,100,120)(H,101,119)(H,102,116)(H,108,109)(H,110,111)(H,112,113)(H,121,122)(H,123,124,125)/t65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.417 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517384

(CHEMBL4443415)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H127N13O28S/c1-3-5-29-63(80(112)91-54-73(102)93-68(50-59-53-90-62-31-25-24-28-61(59)62)83(115)96-64(30-6-4-2)81(113)99-70(52-78(109)110)85(117)97-66(79(87)111)48-57-26-20-19-21-27-57)95-82(114)67(49-58-34-36-60(37-35-58)127-128(120,121)122)98-84(116)69(51-77(107)108)94-75(104)56-126-47-45-124-43-41-89-74(103)55-125-46-44-123-42-40-88-71(100)39-38-65(86(118)119)92-72(101)32-22-17-15-13-11-9-7-8-10-12-14-16-18-23-33-76(105)106/h19-21,24-28,31,34-37,53,63-70,90H,3-18,22-23,29-30,32-33,38-52,54-56H2,1-2H3,(H2,87,111)(H,88,100)(H,89,103)(H,91,112)(H,92,101)(H,93,102)(H,94,104)(H,95,114)(H,96,115)(H,97,117)(H,98,116)(H,99,113)(H,105,106)(H,107,108)(H,109,110)(H,118,119)(H,120,121,122)/t63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517381

(CHEMBL4561184)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C89H131N13O30S/c1-3-5-29-64(82(116)98-66(39-41-78(109)110)84(118)101-70(52-60-55-93-63-31-25-24-28-62(60)63)86(120)97-65(30-6-4-2)83(117)102-72(54-80(113)114)88(122)99-68(81(90)115)50-58-26-20-19-21-27-58)96-85(119)69(51-59-34-36-61(37-35-59)132-133(125,126)127)100-87(121)71(53-79(111)112)95-76(106)57-131-49-47-129-45-43-92-75(105)56-130-48-46-128-44-42-91-73(103)40-38-67(89(123)124)94-74(104)32-22-17-15-13-11-9-7-8-10-12-14-16-18-23-33-77(107)108/h19-21,24-28,31,34-37,55,64-72,93H,3-18,22-23,29-30,32-33,38-54,56-57H2,1-2H3,(H2,90,115)(H,91,103)(H,92,105)(H,94,104)(H,95,106)(H,96,119)(H,97,120)(H,98,116)(H,99,122)(H,100,121)(H,101,118)(H,102,117)(H,107,108)(H,109,110)(H,111,112)(H,113,114)(H,123,124)(H,125,126,127)/t64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517379
(CHEMBL4453982)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(CS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O27S/c1-4-5-28-64(82(114)100-71(51-79(110)111)86(118)97-66(80(88)112)47-58-25-19-18-20-26-58)96-84(116)69(49-61-52-91-63-29-24-23-27-62(61)63)94-74(103)53-92-81(113)67(46-57(2)3)98-83(115)68(48-59-32-34-60(35-33-59)56-128(121,122)123)99-85(117)70(50-78(108)109)95-76(105)55-127-45-43-125-41-39-90-75(104)54-126-44-42-124-40-38-89-72(101)37-36-65(87(119)120)93-73(102)30-21-16-14-12-10-8-6-7-9-11-13-15-17-22-31-77(106)107/h18-20,23-27,29,32-35,52,57,64-71,91H,4-17,21-22,28,30-31,36-51,53-56H2,1-3H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,116)(H,97,118)(H,98,115)(H,99,117)(H,100,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.447 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517370
(CHEMBL4451154)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C97H124N20O31S/c1-49(2)39-68(114-95(146)71(43-54-46-100-59-18-11-9-16-57(54)59)116-97(148)73-19-12-37-117(73)76(121)48-102-85(136)60-24-30-74(119)104-60)93(144)110-65(29-35-81(130)131)91(142)109-64(28-34-80(128)129)90(141)108-63(27-33-79(126)127)89(140)107-62(26-32-78(124)125)88(139)106-61(25-31-77(122)123)87(138)103-50(3)84(135)113-69(41-52-20-22-55(118)23-21-52)86(137)101-47-75(120)105-70(42-53-45-99-58-17-10-8-15-56(53)58)94(145)111-66(36-38-149-4)92(143)115-72(44-82(132)133)96(147)112-67(83(98)134)40-51-13-6-5-7-14-51/h5-11,13-18,20-23,45-46,49-50,60-73,99-100,118H,12,19,24-44,47-48H2,1-4H3,(H2,98,134)(H,101,137)(H,102,136)(H,103,138)(H,104,119)(H,105,120)(H,106,139)(H,107,140)(H,108,141)(H,109,142)(H,110,144)(H,111,145)(H,112,147)(H,113,135)(H,114,146)(H,115,143)(H,116,148)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H,132,133)/t50-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.457 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517339
(CHEMBL4551834)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O28S/c1-5-6-29-64(82(114)99-70(52-79(110)111)86(118)100(4)71(80(88)112)49-58-26-20-19-21-27-58)96-84(116)68(50-60-53-91-63-30-25-24-28-62(60)63)94-74(103)54-92-81(113)66(47-57(2)3)97-83(115)67(48-59-33-35-61(36-34-59)128-129(121,122)123)98-85(117)69(51-78(108)109)95-76(105)56-127-46-44-125-42-40-90-75(104)55-126-45-43-124-41-39-89-72(101)38-37-65(87(119)120)93-73(102)31-22-17-15-13-11-9-7-8-10-12-14-16-18-23-32-77(106)107/h19-21,24-28,30,33-36,53,57,64-71,91H,5-18,22-23,29,31-32,37-52,54-56H2,1-4H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,116)(H,97,115)(H,98,117)(H,99,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.457 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517362

(CHEMBL4565336)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H127N13O28S/c1-4-5-28-63(81(113)99-70(51-78(109)110)85(117)96-65(79(87)111)47-57-25-19-18-20-26-57)95-83(115)68(49-59-52-90-62-29-24-23-27-61(59)62)93-73(102)53-91-80(112)66(46-56(2)3)97-82(114)67(48-58-32-34-60(35-33-58)127-128(120,121)122)98-84(116)69(50-77(107)108)94-75(104)55-126-45-43-124-41-39-89-74(103)54-125-44-42-123-40-38-88-71(100)37-36-64(86(118)119)92-72(101)30-21-16-14-12-10-8-6-7-9-11-13-15-17-22-31-76(105)106/h18-20,23-27,29,32-35,52,56,63-70,90H,4-17,21-22,28,30-31,36-51,53-55H2,1-3H3,(H2,87,111)(H,88,100)(H,89,103)(H,91,112)(H,92,101)(H,93,102)(H,94,104)(H,95,115)(H,96,117)(H,97,114)(H,98,116)(H,99,113)(H,105,106)(H,107,108)(H,109,110)(H,118,119)(H,120,121,122)/t63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517331
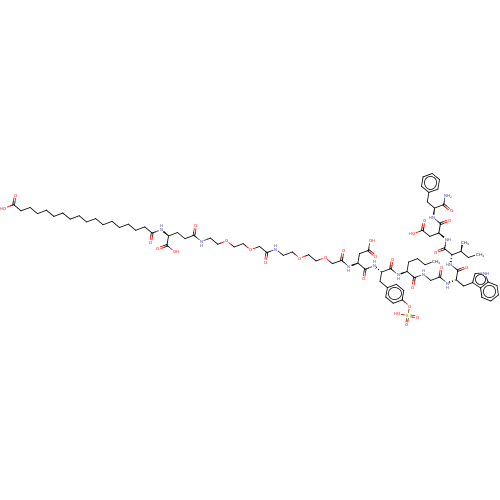
(CHEMBL4467338)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H127N13O28S/c1-4-6-29-63(80(112)91-53-72(102)93-67(49-59-52-90-62-30-25-24-28-61(59)62)84(116)99-78(56(3)5-2)85(117)98-69(51-77(109)110)83(115)96-65(79(87)111)47-57-26-20-19-21-27-57)95-81(113)66(48-58-33-35-60(36-34-58)127-128(120,121)122)97-82(114)68(50-76(107)108)94-74(104)55-126-46-44-124-42-40-89-73(103)54-125-45-43-123-41-39-88-70(100)38-37-64(86(118)119)92-71(101)31-22-17-15-13-11-9-7-8-10-12-14-16-18-23-32-75(105)106/h19-21,24-28,30,33-36,52,56,63-69,78,90H,4-18,22-23,29,31-32,37-51,53-55H2,1-3H3,(H2,87,111)(H,88,100)(H,89,103)(H,91,112)(H,92,101)(H,93,102)(H,94,104)(H,95,113)(H,96,115)(H,97,114)(H,98,117)(H,99,116)(H,105,106)(H,107,108)(H,109,110)(H,118,119)(H,120,121,122)/t56-,63-,64-,65-,66-,67-,68-,69-,78-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517350

(CHEMBL4445353)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C90H136N14O28S/c1-3-5-31-66(83(116)98-68(34-26-27-43-91)85(118)103-72(54-62-57-95-65-33-25-24-30-64(62)65)87(120)100-67(32-6-4-2)84(117)104-74(56-81(113)114)89(122)101-70(82(92)115)52-60-28-20-19-21-29-60)99-86(119)71(53-61-37-39-63(40-38-61)132-133(125,126)127)102-88(121)73(55-80(111)112)97-78(108)59-131-51-49-129-47-45-94-77(107)58-130-50-48-128-46-44-93-75(105)42-41-69(90(123)124)96-76(106)35-22-17-15-13-11-9-7-8-10-12-14-16-18-23-36-79(109)110/h19-21,24-25,28-30,33,37-40,57,66-74,95H,3-18,22-23,26-27,31-32,34-36,41-56,58-59,91H2,1-2H3,(H2,92,115)(H,93,105)(H,94,107)(H,96,106)(H,97,108)(H,98,116)(H,99,119)(H,100,120)(H,101,122)(H,102,121)(H,103,118)(H,104,117)(H,109,110)(H,111,112)(H,113,114)(H,123,124)(H,125,126,127)/t66-,67-,68-,69-,70-,71-,72-,73-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.832 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50154437
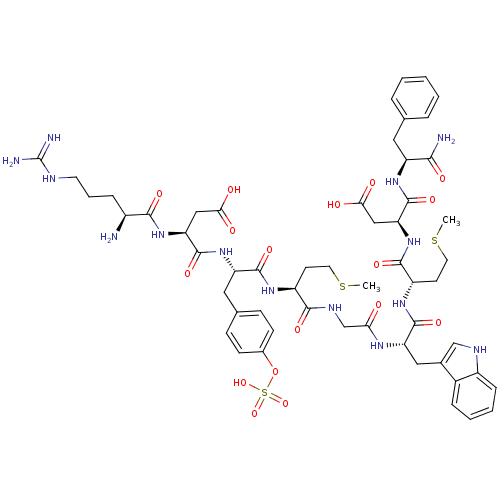
(CHEMBL414345 | Cholecystokinin-9)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H74N14O17S3/c1-87-21-18-37(64-51(79)40(24-31-14-16-33(17-15-31)86-89(83,84)85)68-54(82)42(26-45(71)72)67-48(76)35(56)12-8-20-60-55(58)59)49(77)62-29-44(70)63-41(25-32-28-61-36-13-7-6-11-34(32)36)52(80)65-38(19-22-88-2)50(78)69-43(27-46(73)74)53(81)66-39(47(57)75)23-30-9-4-3-5-10-30/h3-7,9-11,13-17,28,35,37-43,61H,8,12,18-27,29,56H2,1-2H3,(H2,57,75)(H,62,77)(H,63,70)(H,64,79)(H,65,80)(H,66,81)(H,67,76)(H,68,82)(H,69,78)(H,71,72)(H,73,74)(H4,58,59,60)(H,83,84,85)/t35-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of (Thr,Nle)-CCK-9 -induced inositol phosphate production in COS-7 cells expressing human CCK2 receptor |
J Med Chem 47: 5318-29 (2004)
Article DOI: 10.1021/jm0498755
BindingDB Entry DOI: 10.7270/Q2VX0H8G |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517363
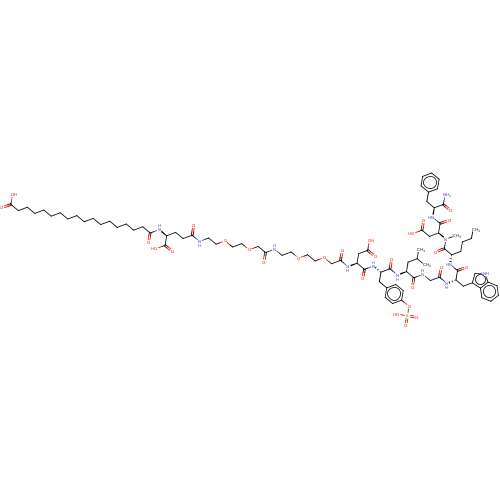
(CHEMBL4471871)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O28S/c1-5-6-29-64(86(118)100(4)71(52-79(110)111)85(117)97-66(80(88)112)48-58-26-20-19-21-27-58)96-83(115)69(50-60-53-91-63-30-25-24-28-62(60)63)94-74(103)54-92-81(113)67(47-57(2)3)98-82(114)68(49-59-33-35-61(36-34-59)128-129(121,122)123)99-84(116)70(51-78(108)109)95-76(105)56-127-46-44-125-42-40-90-75(104)55-126-45-43-124-41-39-89-72(101)38-37-65(87(119)120)93-73(102)31-22-17-15-13-11-9-7-8-10-12-14-16-18-23-32-77(106)107/h19-21,24-28,30,33-36,53,57,64-71,91H,5-18,22-23,29,31-32,37-52,54-56H2,1-4H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,115)(H,97,117)(H,98,114)(H,99,116)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517357

(CHEMBL4568746)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| Show InChI InChI=1S/C91H131N13O28S/c1-4-6-32-68(85(117)96-57-78(107)98-73(53-63-56-95-67-34-25-24-31-66(63)67)87(119)101-69(33-7-5-2)90(122)104(3)75(55-83(114)115)89(121)102-71(84(92)116)52-62-29-26-28-61-27-22-23-30-65(61)62)100-86(118)72(51-60-37-39-64(40-38-60)132-133(125,126)127)103-88(120)74(54-82(112)113)99-80(109)59-131-50-48-129-46-44-94-79(108)58-130-49-47-128-45-43-93-76(105)42-41-70(91(123)124)97-77(106)35-20-18-16-14-12-10-8-9-11-13-15-17-19-21-36-81(110)111/h22-31,34,37-40,56,68-75,95H,4-21,32-33,35-36,41-55,57-59H2,1-3H3,(H2,92,116)(H,93,105)(H,94,108)(H,96,117)(H,97,106)(H,98,107)(H,99,109)(H,100,118)(H,101,119)(H,102,121)(H,103,120)(H,110,111)(H,112,113)(H,114,115)(H,123,124)(H,125,126,127)/t68-,69-,70-,71-,72-,73-,74-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50600026
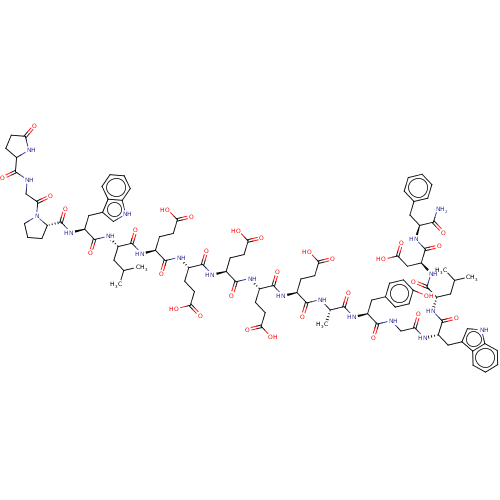
(CHEMBL5177923)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)C1CCC(=O)N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114214
BindingDB Entry DOI: 10.7270/Q2GM8CCP |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.66 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Activity at CCK2R-MOPR (unknown origin) coexpressed in CHO cells cotransfected with delta6-Galphaqi4-myr assessed as intracellular calcium release by... |
J Med Chem 52: 247-58 (2009)
Article DOI: 10.1021/jm800174p
BindingDB Entry DOI: 10.7270/Q22V2FZJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517342
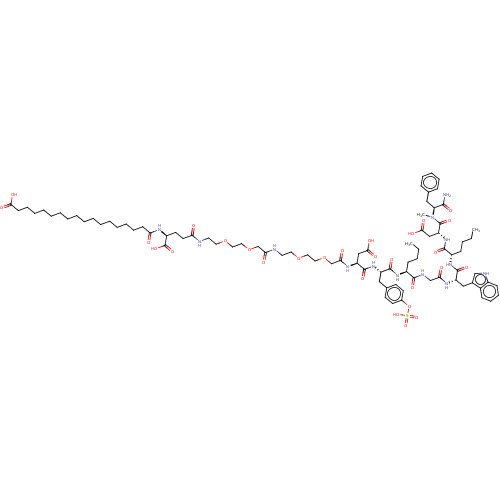
(CHEMBL4591101)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C87H129N13O28S/c1-4-6-30-64(81(113)92-55-74(103)94-68(51-60-54-91-63-32-26-25-29-62(60)63)84(116)97-65(31-7-5-2)82(114)99-70(53-79(110)111)86(118)100(3)71(80(88)112)50-58-27-21-20-22-28-58)96-83(115)67(49-59-35-37-61(38-36-59)128-129(121,122)123)98-85(117)69(52-78(108)109)95-76(105)57-127-48-46-125-44-42-90-75(104)56-126-47-45-124-43-41-89-72(101)40-39-66(87(119)120)93-73(102)33-23-18-16-14-12-10-8-9-11-13-15-17-19-24-34-77(106)107/h20-22,25-29,32,35-38,54,64-71,91H,4-19,23-24,30-31,33-34,39-53,55-57H2,1-3H3,(H2,88,112)(H,89,101)(H,90,104)(H,92,113)(H,93,102)(H,94,103)(H,95,105)(H,96,115)(H,97,116)(H,98,117)(H,99,114)(H,106,107)(H,108,109)(H,110,111)(H,119,120)(H,121,122,123)/t64-,65-,66-,67-,68-,69-,70+,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21140
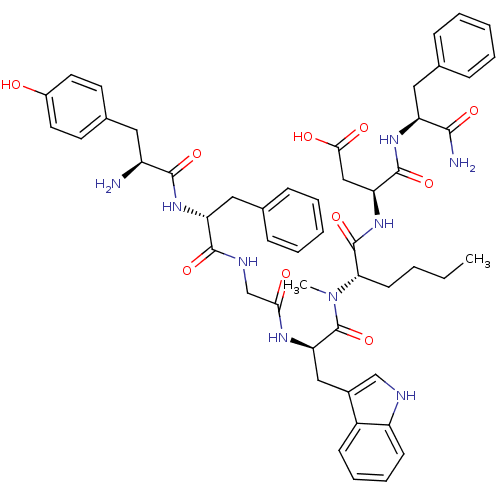
((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H61N9O10/c1-3-4-19-43(50(69)59-41(28-45(63)64)49(68)57-39(46(53)65)25-31-13-7-5-8-14-31)60(2)51(70)42(27-34-29-54-38-18-12-11-17-36(34)38)56-44(62)30-55-48(67)40(26-32-15-9-6-10-16-32)58-47(66)37(52)24-33-20-22-35(61)23-21-33/h5-18,20-23,29,37,39-43,54,61H,3-4,19,24-28,30,52H2,1-2H3,(H2,53,65)(H,55,67)(H,56,62)(H,57,68)(H,58,66)(H,59,69)(H,63,64)/t37-,39-,40+,41-,42+,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50024321
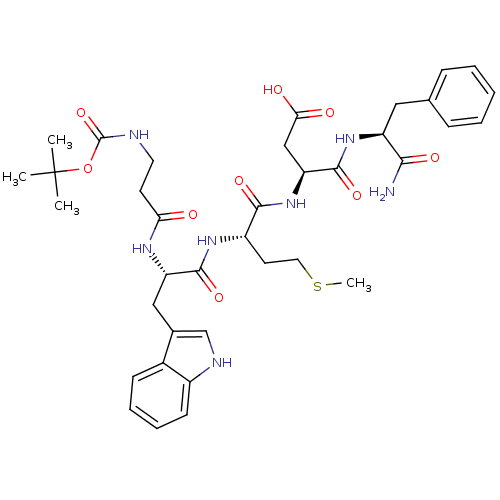
(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21132
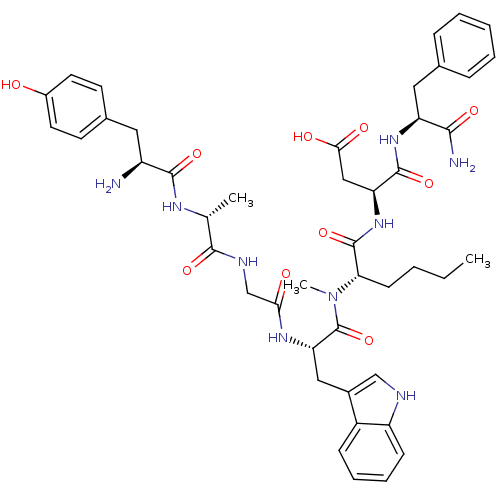
((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H57N9O10/c1-4-5-15-37(44(63)53-35(23-39(57)58)43(62)52-34(40(47)59)21-27-11-7-6-8-12-27)54(3)45(64)36(22-29-24-48-33-14-10-9-13-31(29)33)51-38(56)25-49-41(60)26(2)50-42(61)32(46)20-28-16-18-30(55)19-17-28/h6-14,16-19,24,26,32,34-37,48,55H,4-5,15,20-23,25,46H2,1-3H3,(H2,47,59)(H,49,60)(H,50,61)(H,51,56)(H,52,62)(H,53,63)(H,57,58)/t26-,32+,34+,35+,36+,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517355
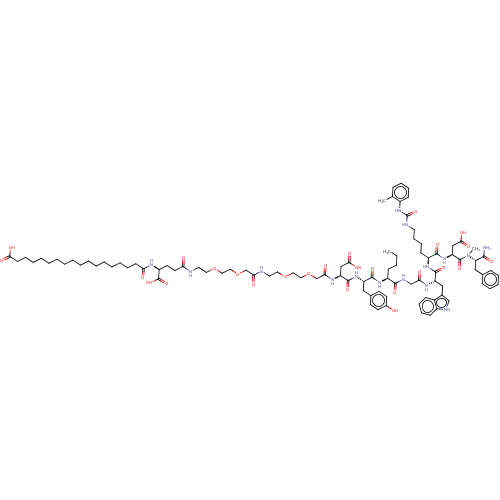
(CHEMBL4471525)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCNC(=O)Nc1ccccc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C95H137N15O26/c1-4-5-33-71(88(124)101-60-81(114)103-75(56-66-59-100-70-35-26-24-32-68(66)70)91(127)106-72(36-27-28-45-99-95(132)109-69-34-25-23-29-63(69)2)89(125)108-77(58-86(121)122)93(129)110(3)78(87(96)123)55-64-30-19-18-20-31-64)105-90(126)74(54-65-39-41-67(111)42-40-65)107-92(128)76(57-85(119)120)104-83(116)62-136-53-51-134-49-47-98-82(115)61-135-52-50-133-48-46-97-79(112)44-43-73(94(130)131)102-80(113)37-21-16-14-12-10-8-6-7-9-11-13-15-17-22-38-84(117)118/h18-20,23-26,29-32,34-35,39-42,59,71-78,100,111H,4-17,21-22,27-28,33,36-38,43-58,60-62H2,1-3H3,(H2,96,123)(H,97,112)(H,98,115)(H,101,124)(H,102,113)(H,103,114)(H,104,116)(H,105,126)(H,106,127)(H,107,128)(H,108,125)(H,117,118)(H,119,120)(H,121,122)(H,130,131)(H2,99,109,132)/t71-,72-,73-,74-,75-,76-,77-,78-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50517337

(CHEMBL4465747)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C86H128N14O27S/c1-3-5-29-63(80(112)92-54-74(104)94-68(50-59-53-91-62-31-25-24-28-61(59)62)83(115)97-64(30-6-4-2)81(113)100-69(51-71(87)101)84(116)98-66(79(88)111)48-57-26-20-19-21-27-57)96-82(114)67(49-58-34-36-60(37-35-58)127-128(120,121)122)99-85(117)70(52-78(109)110)95-76(106)56-126-47-45-124-43-41-90-75(105)55-125-46-44-123-42-40-89-72(102)39-38-65(86(118)119)93-73(103)32-22-17-15-13-11-9-7-8-10-12-14-16-18-23-33-77(107)108/h19-21,24-28,31,34-37,53,63-70,91H,3-18,22-23,29-30,32-33,38-52,54-56H2,1-2H3,(H2,87,101)(H2,88,111)(H,89,102)(H,90,105)(H,92,112)(H,93,103)(H,94,104)(H,95,106)(H,96,114)(H,97,115)(H,98,116)(H,99,117)(H,100,113)(H,107,108)(H,109,110)(H,118,119)(H,120,121,122)/t63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK2R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assay |
J Med Chem 62: 1407-1419 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01558
BindingDB Entry DOI: 10.7270/Q2K64NF5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004436

(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN(C)C(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(49(72)63-44(28-47(69)70)53(76)61-40(48(55)71)24-32-14-10-9-11-15-32)59-51(74)42(26-34-29-56-37-19-13-12-16-36(34)37)58-45(66)30-64(4)54(77)39(18-8-6-2)60-50(73)41(62-52(75)43(27-46(67)68)57-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,65)(H,58,66)(H,59,74)(H,60,73)(H,61,76)(H,62,75)(H,63,72)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21141
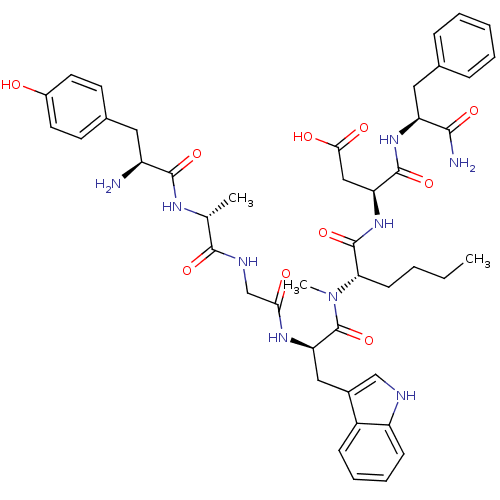
((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H57N9O10/c1-4-5-15-37(44(63)53-35(23-39(57)58)43(62)52-34(40(47)59)21-27-11-7-6-8-12-27)54(3)45(64)36(22-29-24-48-33-14-10-9-13-31(29)33)51-38(56)25-49-41(60)26(2)50-42(61)32(46)20-28-16-18-30(55)19-17-28/h6-14,16-19,24,26,32,34-37,48,55H,4-5,15,20-23,25,46H2,1-3H3,(H2,47,59)(H,49,60)(H,50,61)(H,51,56)(H,52,62)(H,53,63)(H,57,58)/t26-,32+,34+,35+,36-,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554548

(CHEMBL4788440)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCCCCNC(=O)C1CCCCNC(=O)CCN2C(=O)CC(SC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc3c[nH]cn3)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CSC3CC(=O)N(CCC(=O)N1)C3=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C2=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554546
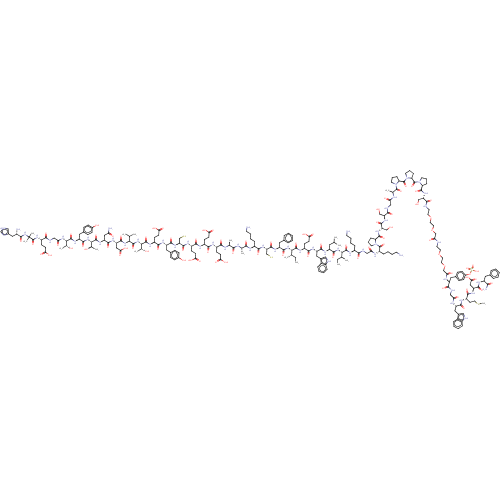
(CHEMBL4780789)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc1c[nH]cn1)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21138
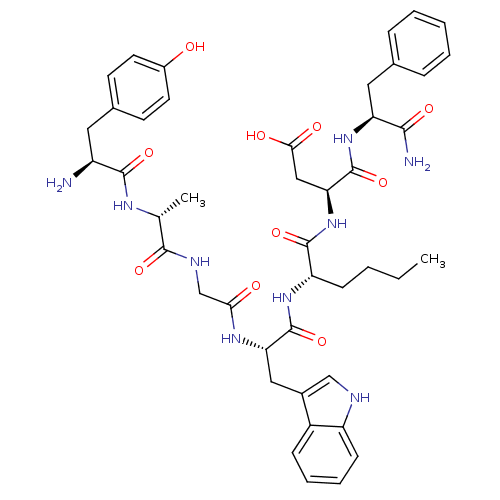
((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C44H55N9O10/c1-3-4-13-33(42(61)53-36(22-38(56)57)44(63)52-34(39(46)58)20-26-10-6-5-7-11-26)51-43(62)35(21-28-23-47-32-14-9-8-12-30(28)32)50-37(55)24-48-40(59)25(2)49-41(60)31(45)19-27-15-17-29(54)18-16-27/h5-12,14-18,23,25,31,33-36,47,54H,3-4,13,19-22,24,45H2,1-2H3,(H2,46,58)(H,48,59)(H,49,60)(H,50,55)(H,51,62)(H,52,63)(H,53,61)(H,56,57)/t25-,31+,33+,34+,35+,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554547
(CHEMBL4784851)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc1c[nH]cn1)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554544
(CHEMBL4783736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc1c[nH]cn1)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554549
(CHEMBL4795718)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCCCCNC(=O)C1CCCCNC(=O)CCN2C(=O)CC(SC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc3c[nH]cn3)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CSC3CC(=O)N(CCC(=O)N1)C3=O)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O)C2=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004437
(3-Acetylamino-N-[1-[1-({[1-(1-{[1-(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(60-50(73)41(63-52(75)43(27-46(67)68)58-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80)49(72)57-30-45(66)59-42(26-34-29-56-37-19-13-12-16-36(34)37)51(74)61-39(18-8-6-2)54(77)64(4)44(28-47(69)70)53(76)62-40(48(55)71)24-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,73)(H,61,74)(H,62,76)(H,63,75)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554545

(CHEMBL4799182)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc1c[nH]cn1)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554555
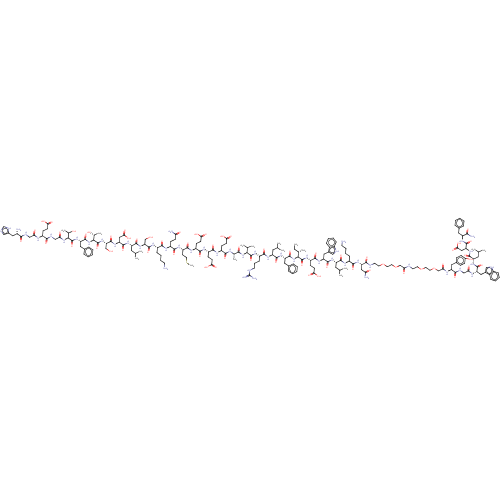
(CHEMBL4741697)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004439
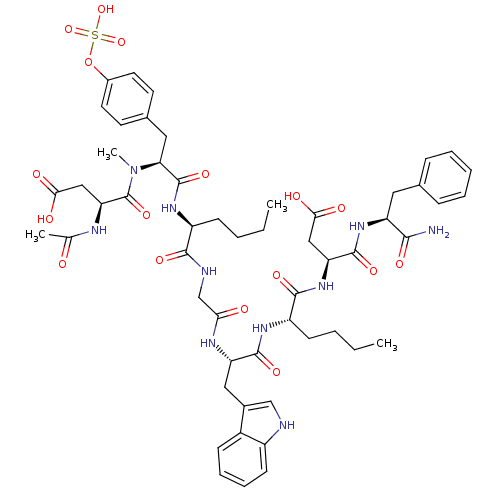
(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)N(C)C(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(61-53(76)44(25-33-20-22-35(23-21-33)81-82(78,79)80)64(4)54(77)43(28-47(69)70)58-31(3)65)49(72)57-30-45(66)59-41(26-34-29-56-37-19-13-12-16-36(34)37)51(74)60-39(18-8-6-2)50(73)63-42(27-46(67)68)52(75)62-40(48(55)71)24-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,74)(H,61,76)(H,62,75)(H,63,73)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50549226
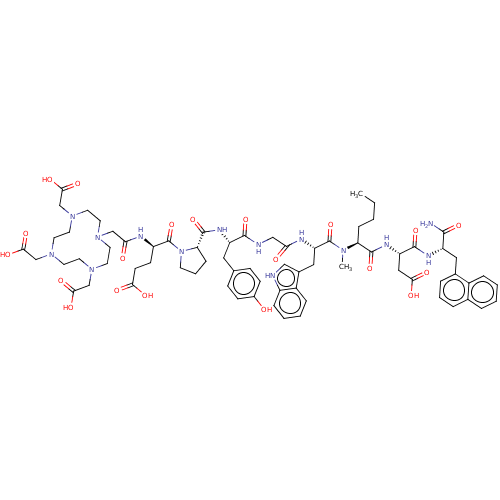
(CHEMBL4795913)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50549228
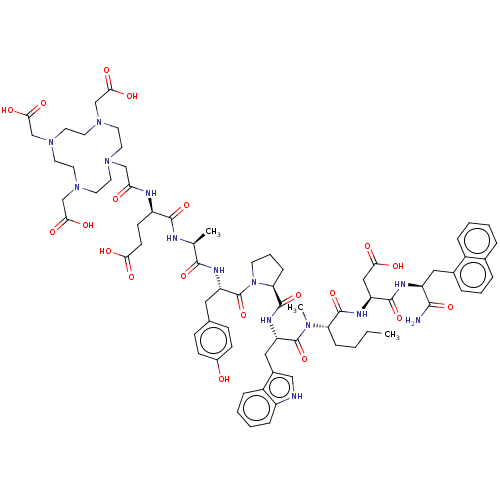
(CHEMBL4758706)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50002477

((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H44N6O8S/c1-34(2,3)48-33(47)40-26(17-21-19-36-23-13-9-8-12-22(21)23)31(45)37-24(14-15-49-4)30(44)39-27(18-28(41)42)32(46)38-25(29(35)43)16-20-10-6-5-7-11-20/h5-13,19,24-27,36H,14-18H2,1-4H3,(H2,35,43)(H,37,45)(H,38,46)(H,39,44)(H,40,47)(H,41,42)/t24-,25-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at CCKBR in human NCI-H345 cells assessed as calcium release |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00530
BindingDB Entry DOI: 10.7270/Q2W95DW9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50549227
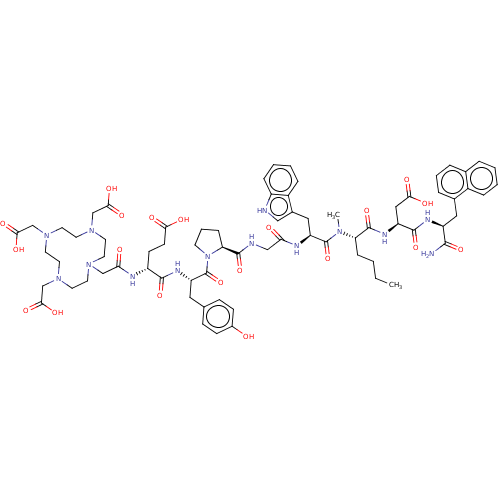
(CHEMBL4759105)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CCC(O)=O)NC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1cccc2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK2R expressed in human A431 cells assessed as intracellular calcium mobilization measured after 24 hrs by Fluo-4AM dye ba... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01233
BindingDB Entry DOI: 10.7270/Q2P84GJ2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004440
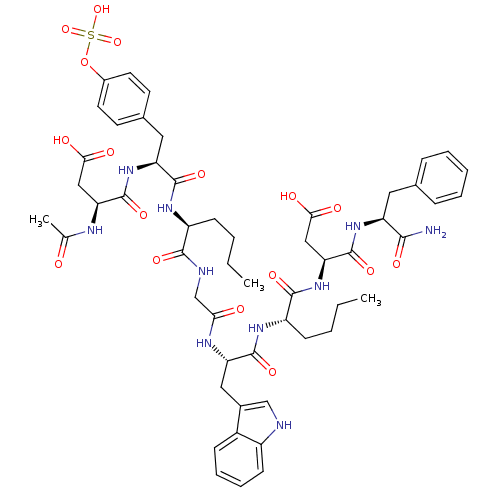
(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H68N10O17S/c1-4-6-16-37(59-50(73)40(62-52(75)42(26-45(66)67)57-30(3)64)24-32-19-21-34(22-20-32)80-81(77,78)79)48(71)56-29-44(65)58-41(25-33-28-55-36-18-12-11-15-35(33)36)51(74)60-38(17-7-5-2)49(72)63-43(27-46(68)69)53(76)61-39(47(54)70)23-31-13-9-8-10-14-31/h8-15,18-22,28,37-43,55H,4-7,16-17,23-27,29H2,1-3H3,(H2,54,70)(H,56,71)(H,57,64)(H,58,65)(H,59,73)(H,60,74)(H,61,76)(H,62,75)(H,63,72)(H,66,67)(H,68,69)(H,77,78,79)/t37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50554550
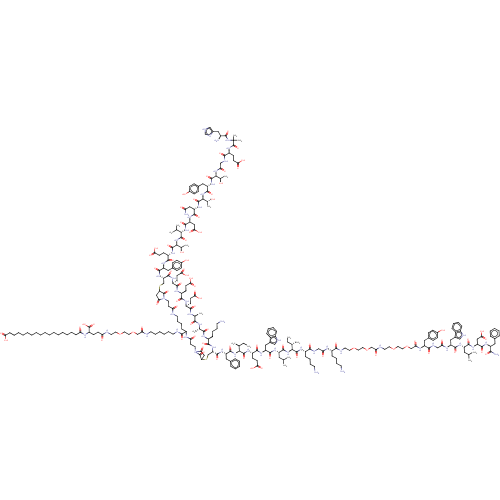
(CHEMBL4764500)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CSC2CC(=O)N(CCC(=O)NC(CCCCNC(=O)CCN3C(=O)CC(SC[C@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)Cc4c[nH]cn4)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N1)C3=O)C(=O)NCCCCCCNC(=O)COCCOCCNC(=O)CCC(NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)C2=O)[C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCCOCCOCC(=O)NCCOCCOCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human CCK-2R expressed in HEK293 cells assessed as stimulation of ERK phosphorylation incubated for 5 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00736
BindingDB Entry DOI: 10.7270/Q25H7KX4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21131
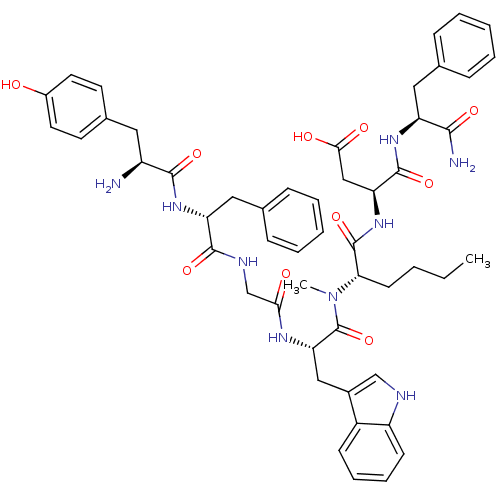
((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H61N9O10/c1-3-4-19-43(50(69)59-41(28-45(63)64)49(68)57-39(46(53)65)25-31-13-7-5-8-14-31)60(2)51(70)42(27-34-29-54-38-18-12-11-17-36(34)38)56-44(62)30-55-48(67)40(26-32-15-9-6-10-16-32)58-47(66)37(52)24-33-20-22-35(61)23-21-33/h5-18,20-23,29,37,39-43,54,61H,3-4,19,24-28,30,52H2,1-2H3,(H2,53,65)(H,55,67)(H,56,62)(H,57,68)(H,58,66)(H,59,69)(H,63,64)/t37-,39-,40+,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50202108

((3S)-3-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1cc2ccccc2[nH]1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C58H73N11O13/c1-6-7-21-43(64-53(77)46(67-57(81)82-58(3,4)5)31-39-30-38-20-14-15-22-42(38)62-39)52(76)66-47(32-49(72)73)54(78)65-45(29-36-18-12-9-13-19-36)56(80)69-68-55(79)44(28-35-16-10-8-11-17-35)63-48(71)33-60-50(74)34(2)61-51(75)41(59)27-37-23-25-40(70)26-24-37/h8-20,22-26,30,34,41,43-47,62,70H,6-7,21,27-29,31-33,59H2,1-5H3,(H,60,74)(H,61,75)(H,63,71)(H,64,77)(H,65,78)(H,66,76)(H,67,81)(H,68,79)(H,69,80)(H,72,73)/t34-,41+,43+,44+,45+,46-,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cells |
J Med Chem 50: 165-8 (2007)
Article DOI: 10.1021/jm061268p
BindingDB Entry DOI: 10.7270/Q2TD9X17 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data