 Found 348 hits of ec50 data for polymerid = 2130,49000160,49000161
Found 348 hits of ec50 data for polymerid = 2130,49000160,49000161 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50451378
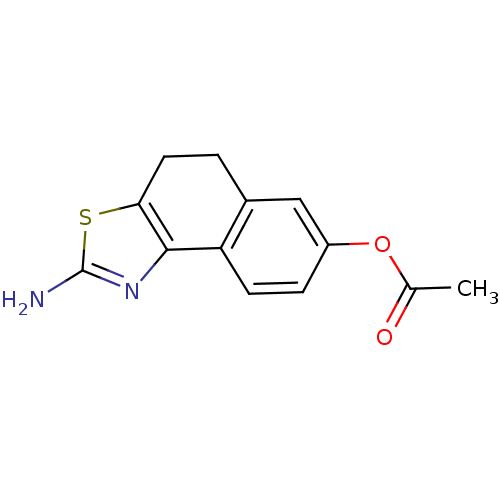
(CHEMBL39106)Show InChI InChI=1S/C13H12N2O2S.HI/c1-7(16)17-9-3-4-10-8(6-9)2-5-11-12(10)15-13(14)18-11;/h3-4,6H,2,5H2,1H3,(H2,14,15);1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
AE maximal score at Adenosine A2A receptor |
Bioorg Med Chem Lett 12: 1563-6 (2002)
BindingDB Entry DOI: 10.7270/Q2833RBV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50451381
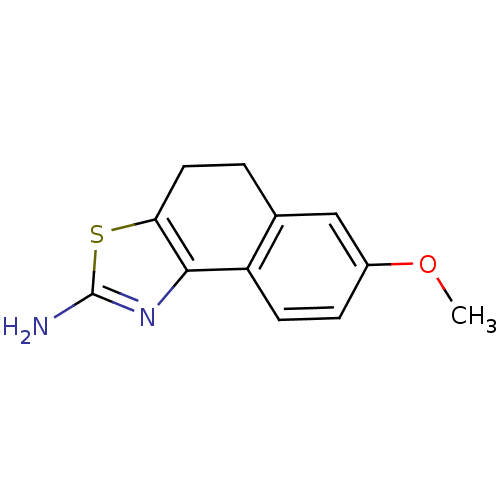
(CHEMBL38779)Show InChI InChI=1S/C12H12N2OS.HI/c1-15-8-3-4-9-7(6-8)2-5-10-11(9)14-12(13)16-10;/h3-4,6H,2,5H2,1H3,(H2,13,14);1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
AE maximal score at Adenosine A2A receptor |
Bioorg Med Chem Lett 12: 1563-6 (2002)
BindingDB Entry DOI: 10.7270/Q2833RBV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50451379
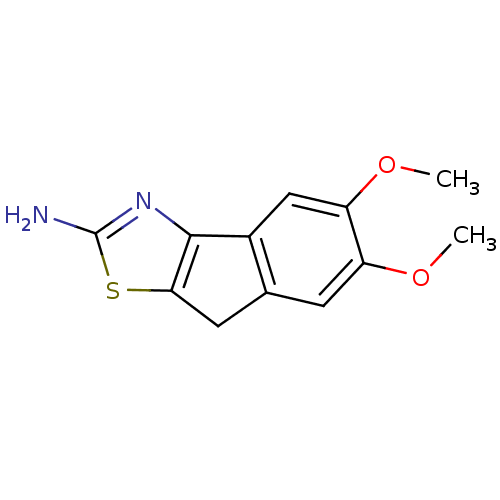
(CHEMBL43299)Show InChI InChI=1S/C12H12N2O2S.HI/c1-15-8-3-6-4-10-11(14-12(13)17-10)7(6)5-9(8)16-2;/h3,5H,4H2,1-2H3,(H2,13,14);1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
AE maximal score at Adenosine A2A receptor |
Bioorg Med Chem Lett 12: 1563-6 (2002)
BindingDB Entry DOI: 10.7270/Q2833RBV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513547
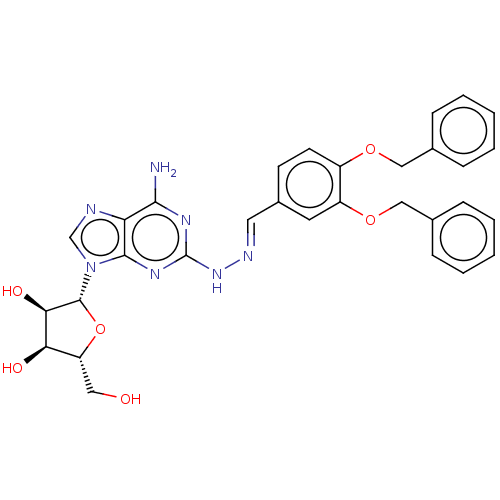
(CHEMBL4471366)Show SMILES Nc1nc(N\N=C\c2ccc(OCc3ccccc3)c(OCc3ccccc3)c2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C31H31N7O6/c32-28-25-29(38(18-33-25)30-27(41)26(40)24(15-39)44-30)36-31(35-28)37-34-14-21-11-12-22(42-16-19-7-3-1-4-8-19)23(13-21)43-17-20-9-5-2-6-10-20/h1-14,18,24,26-27,30,39-41H,15-17H2,(H3,32,35,36,37)/b34-14+/t24-,26-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50385948
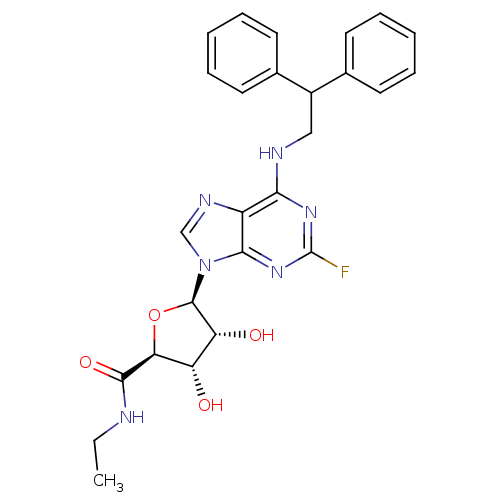
(CHEMBL2042304)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(F)nc12 |r| Show InChI InChI=1S/C26H27FN6O4/c1-2-28-24(36)21-19(34)20(35)25(37-21)33-14-30-18-22(31-26(27)32-23(18)33)29-13-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,14,17,19-21,25,34-35H,2,13H2,1H3,(H,28,36)(H,29,31,32)/t19-,20+,21-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins |
J Med Chem 55: 3521-34 (2012)
Article DOI: 10.1021/jm300206u
BindingDB Entry DOI: 10.7270/Q2FT8N35 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50595579
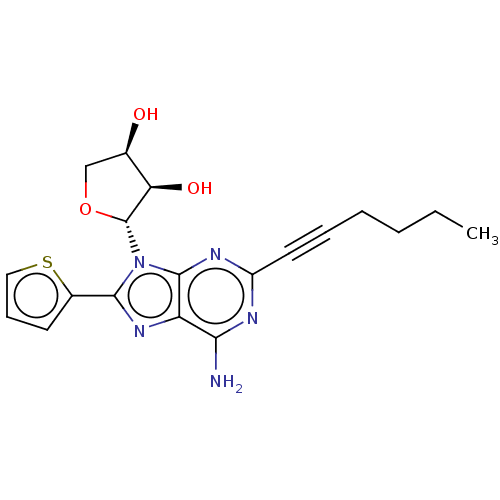
(CHEMBL5203469)Show SMILES CCCCC#Cc1nc(N)c2nc(-c3cccs3)n([C@@H]3OC[C@@H](O)[C@H]3O)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00462
BindingDB Entry DOI: 10.7270/Q2NV9P8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150766
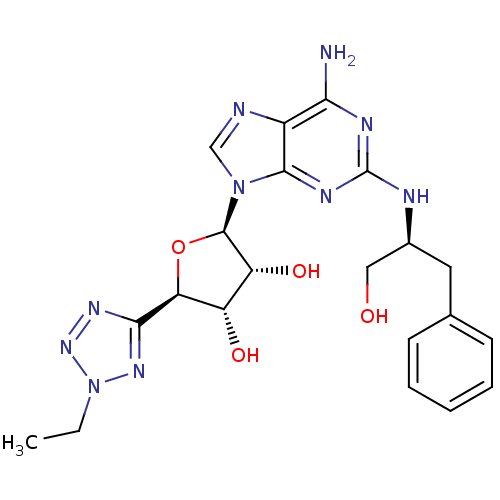
((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccccc3)nc12 Show InChI InChI=1S/C21H26N10O4/c1-2-31-28-18(27-29-31)16-14(33)15(34)20(35-16)30-10-23-13-17(22)25-21(26-19(13)30)24-12(9-32)8-11-6-4-3-5-7-11/h3-7,10,12,14-16,20,32-34H,2,8-9H2,1H3,(H3,22,24,25,26)/t12-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor expressed in CHO cells assessed as induction of cyclic AMP production |
J Med Chem 55: 5676-703 (2012)
Article DOI: 10.1021/jm300087j
BindingDB Entry DOI: 10.7270/Q25H7HDK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513551
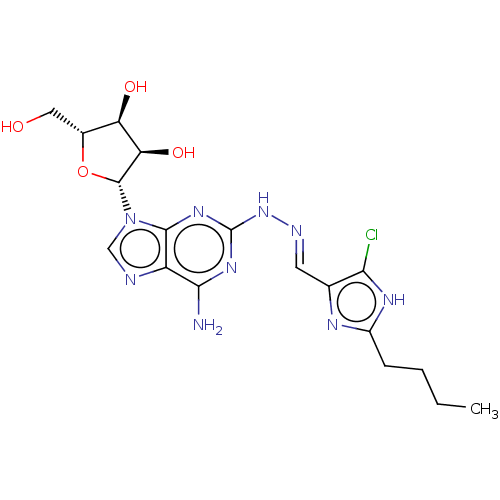
(CHEMBL4447310)Show SMILES CCCCc1nc(\C=N\Nc2nc(N)c3ncn([C@@H]4O[C@H](CO)[C@@H](O)[C@H]4O)c3n2)c(Cl)[nH]1 |r| Show InChI InChI=1S/C18H24ClN9O4/c1-2-3-4-10-23-8(14(19)24-10)5-22-27-18-25-15(20)11-16(26-18)28(7-21-11)17-13(31)12(30)9(6-29)32-17/h5,7,9,12-13,17,29-31H,2-4,6H2,1H3,(H,23,24)(H3,20,25,26,27)/b22-5+/t9-,12-,13-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600755
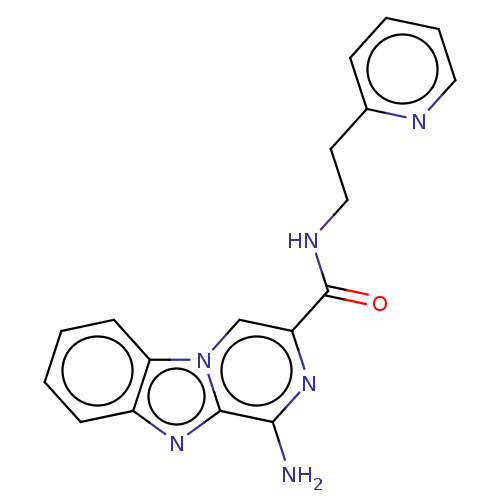
(CHEMBL5171148) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150762
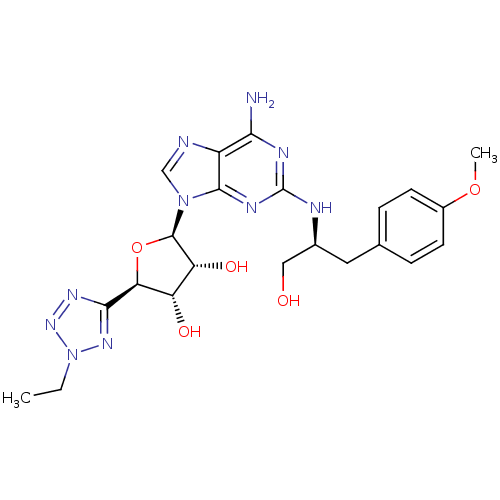
((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-1-hydroxymethyl-2-...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccc(OC)cc3)nc12 Show InChI InChI=1S/C22H28N10O5/c1-3-32-29-19(28-30-32)17-15(34)16(35)21(37-17)31-10-24-14-18(23)26-22(27-20(14)31)25-12(9-33)8-11-4-6-13(36-2)7-5-11/h4-7,10,12,15-17,21,33-35H,3,8-9H2,1-2H3,(H3,23,25,26,27)/t12-,15-,16+,17-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
IIQAB (CSIC)
Curated by ChEMBL
| Assay Description
Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor |
J Med Chem 47: 4041-53 (2004)
Article DOI: 10.1021/jm031143+
BindingDB Entry DOI: 10.7270/Q26D5TR9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Partial agonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as intrinsic activity by measuring cell index change withi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513554
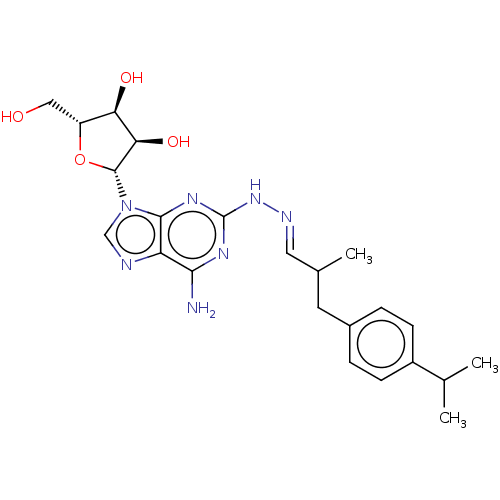
(CHEMBL4438487)Show SMILES CC(Cc1ccc(cc1)C(C)C)\C=N\Nc1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C23H31N7O4/c1-12(2)15-6-4-14(5-7-15)8-13(3)9-26-29-23-27-20(24)17-21(28-23)30(11-25-17)22-19(33)18(32)16(10-31)34-22/h4-7,9,11-13,16,18-19,22,31-33H,8,10H2,1-3H3,(H3,24,27,28,29)/b26-9+/t13?,16-,18-,19-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600757
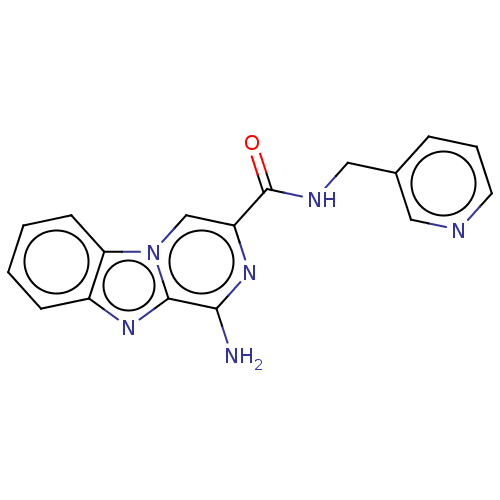
(CHEMBL5208415) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600758
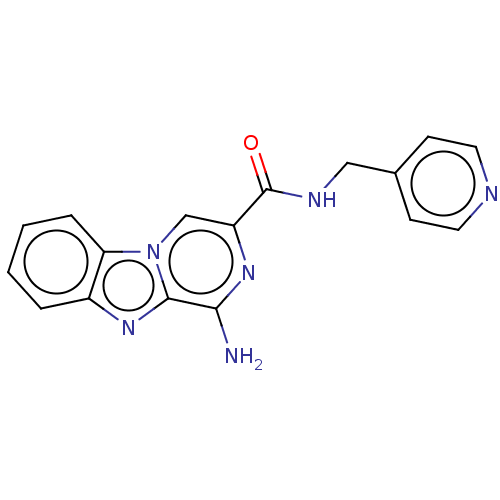
(CHEMBL5174275) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150765
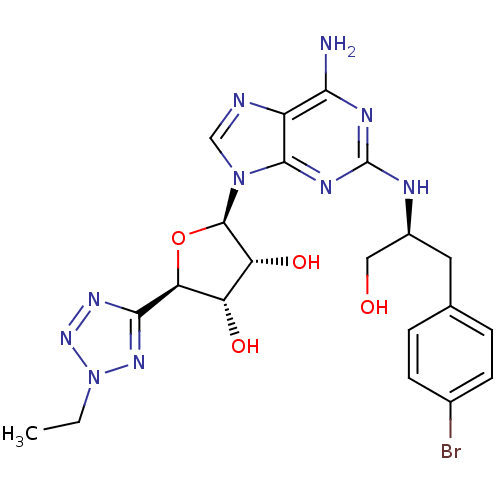
((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-2-(4-bromo-phenyl)...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccc(Br)cc3)nc12 Show InChI InChI=1S/C21H25BrN10O4/c1-2-32-29-18(28-30-32)16-14(34)15(35)20(36-16)31-9-24-13-17(23)26-21(27-19(13)31)25-12(8-33)7-10-3-5-11(22)6-4-10/h3-6,9,12,14-16,20,33-35H,2,7-8H2,1H3,(H3,23,25,26,27)/t12-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
IIQAB (CSIC)
Curated by ChEMBL
| Assay Description
Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor |
J Med Chem 47: 4041-53 (2004)
Article DOI: 10.1021/jm031143+
BindingDB Entry DOI: 10.7270/Q26D5TR9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50600748
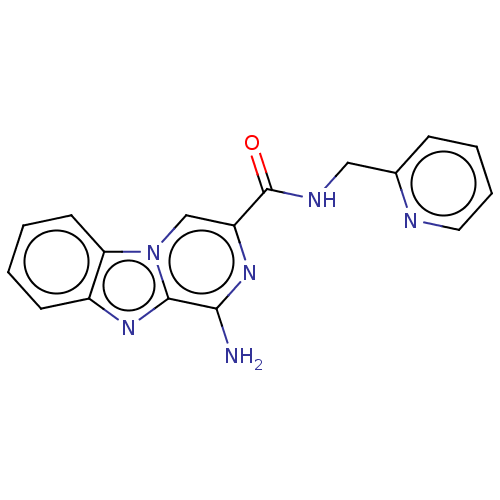
(CHEMBL5176737) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00101
BindingDB Entry DOI: 10.7270/Q2417231 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50385955
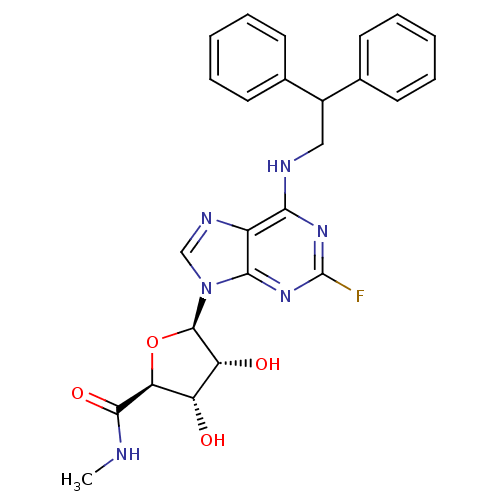
(CHEMBL2042311)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(F)nc12 |r| Show InChI InChI=1S/C25H25FN6O4/c1-27-23(35)20-18(33)19(34)24(36-20)32-13-29-17-21(30-25(26)31-22(17)32)28-12-16(14-8-4-2-5-9-14)15-10-6-3-7-11-15/h2-11,13,16,18-20,24,33-34H,12H2,1H3,(H,27,35)(H,28,30,31)/t18-,19+,20-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.02 | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins |
J Med Chem 55: 3521-34 (2012)
Article DOI: 10.1021/jm300206u
BindingDB Entry DOI: 10.7270/Q2FT8N35 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2a receptor expressed in CHO cells assessed as stimulation of cAMP formation after 24 hrs |
ACS Med Chem Lett 5: 1043-8 (2014)
Article DOI: 10.1021/ml5002486
BindingDB Entry DOI: 10.7270/Q2VD712G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078422
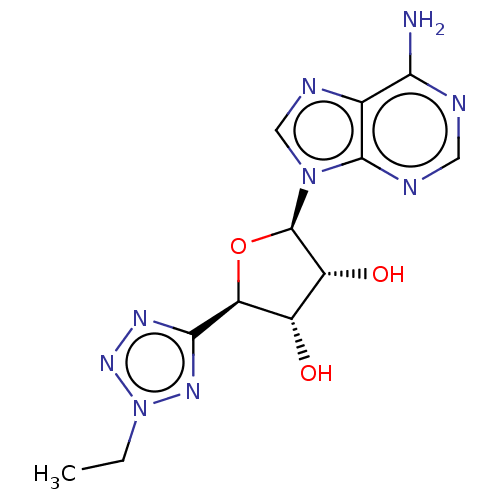
(CHEMBL3414936)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H15N9O3/c1-2-21-18-10(17-19-21)8-6(22)7(23)12(24-8)20-4-16-5-9(13)14-3-15-11(5)20/h3-4,6-8,12,22-23H,2H2,1H3,(H2,13,14,15)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Irreversible agonist activity at human adenosine A2A receptor expressed in Flp-In-CHO cells assessed as effect on forskolin-induced cAMP accumulation... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01561
BindingDB Entry DOI: 10.7270/Q2K93C7T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266652
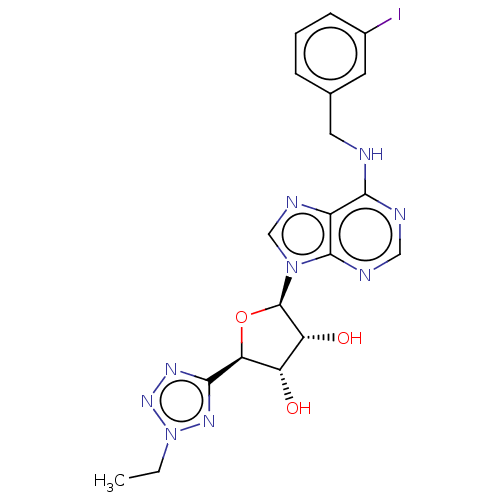
(CHEMBL4078479)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C19H20IN9O3/c1-2-29-26-17(25-27-29)15-13(30)14(31)19(32-15)28-9-24-12-16(22-8-23-18(12)28)21-7-10-4-3-5-11(20)6-10/h3-6,8-9,13-15,19,30-31H,2,7H2,1H3,(H,21,22,23)/t13-,14+,15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human adenosine A2A receptor expressed in CHO cell membranes assessed as stimulation of adenylyl cyclase activity by ... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50003647
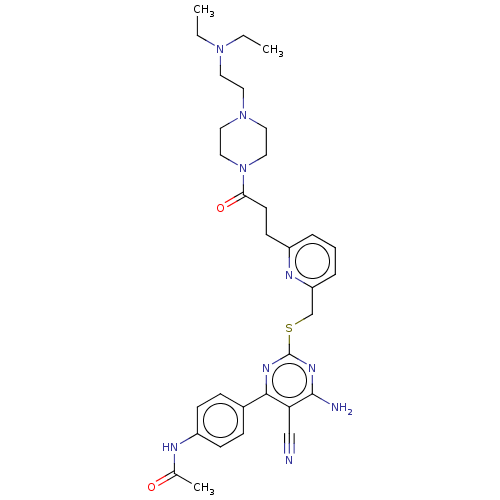
(CHEMBL3235280)Show SMILES CCN(CC)CCN1CCN(CC1)C(=O)CCc1cccc(CSc2nc(N)c(C#N)c(n2)-c2ccc(NC(C)=O)cc2)n1 Show InChI InChI=1S/C32H41N9O2S/c1-4-39(5-2)15-16-40-17-19-41(20-18-40)29(43)14-13-25-7-6-8-27(36-25)22-44-32-37-30(28(21-33)31(34)38-32)24-9-11-26(12-10-24)35-23(3)42/h6-12H,4-5,13-20,22H2,1-3H3,(H,35,42)(H2,34,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in HEK293 cells assessed as increase in intracellular cAMP level after 30 mins by EIA |
J Med Chem 57: 3213-22 (2014)
Article DOI: 10.1021/jm401643m
BindingDB Entry DOI: 10.7270/Q27P90X6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150764
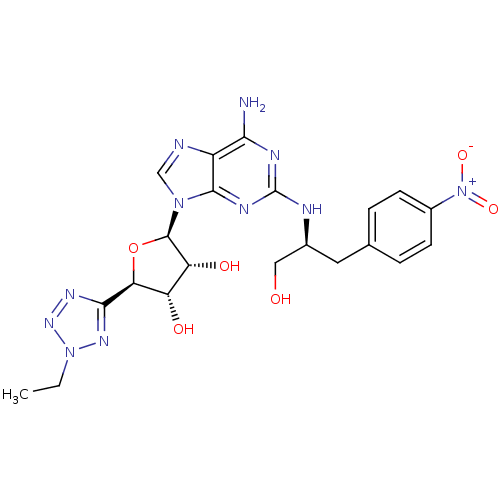
((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-1-hydroxymethyl-2-...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccc(cc3)[N+]([O-])=O)nc12 Show InChI InChI=1S/C21H25N11O6/c1-2-31-28-18(27-29-31)16-14(34)15(35)20(38-16)30-9-23-13-17(22)25-21(26-19(13)30)24-11(8-33)7-10-3-5-12(6-4-10)32(36)37/h3-6,9,11,14-16,20,33-35H,2,7-8H2,1H3,(H3,22,24,25,26)/t11-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
IIQAB (CSIC)
Curated by ChEMBL
| Assay Description
Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor |
J Med Chem 47: 4041-53 (2004)
Article DOI: 10.1021/jm031143+
BindingDB Entry DOI: 10.7270/Q26D5TR9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences , Monash University , Parkville , Victoria 3052 , Australia.
Curated by ChEMBL
| Assay Description
Agonist activity at human A2A-AR expressed in FlpIn-CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation preincubated for 40 mi... |
J Med Chem 61: 2087-2103 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00047
BindingDB Entry DOI: 10.7270/Q21J9D7Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078423
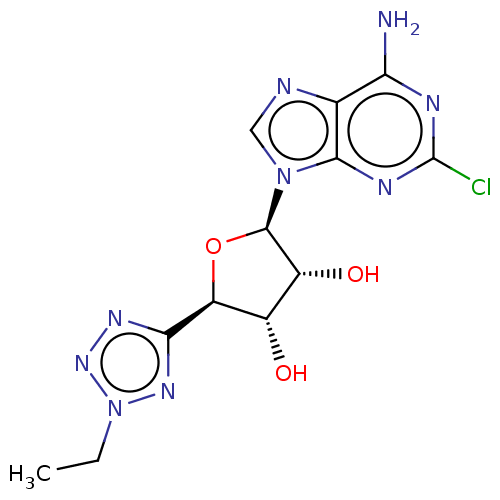
(CHEMBL3414937)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(Cl)nc12 |r| Show InChI InChI=1S/C12H14ClN9O3/c1-2-22-19-9(18-20-22)7-5(23)6(24)11(25-7)21-3-15-4-8(14)16-12(13)17-10(4)21/h3,5-7,11,23-24H,2H2,1H3,(H2,14,16,17)/t5-,6+,7-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513545
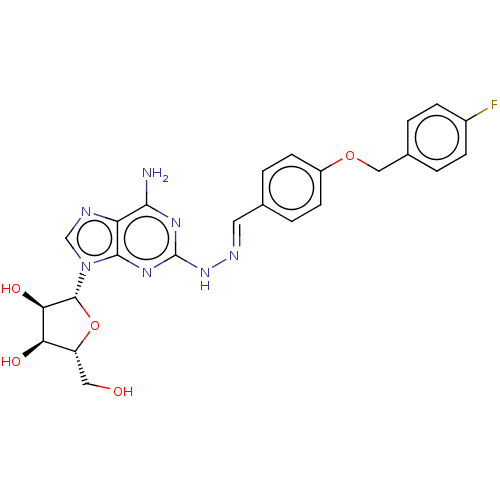
(CHEMBL4576915)Show SMILES Nc1nc(N\N=C\c2ccc(OCc3ccc(F)cc3)cc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H24FN7O5/c25-15-5-1-14(2-6-15)11-36-16-7-3-13(4-8-16)9-28-31-24-29-21(26)18-22(30-24)32(12-27-18)23-20(35)19(34)17(10-33)37-23/h1-9,12,17,19-20,23,33-35H,10-11H2,(H3,26,29,30,31)/b28-9+/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513528
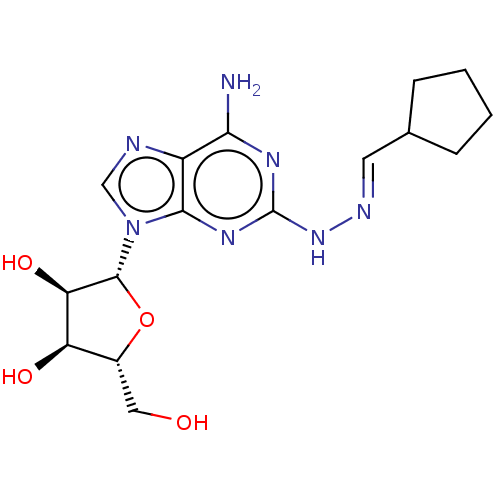
(CHEMBL4440214)Show SMILES Nc1nc(N\N=C\C2CCCC2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H23N7O4/c17-13-10-14(21-16(20-13)22-19-5-8-3-1-2-4-8)23(7-18-10)15-12(26)11(25)9(6-24)27-15/h5,7-9,11-12,15,24-26H,1-4,6H2,(H3,17,20,21,22)/b19-5+/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266651
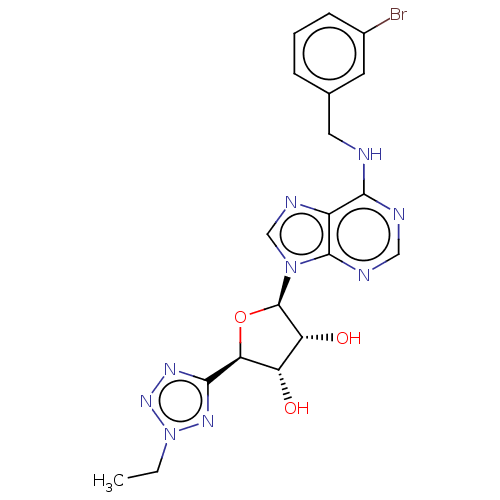
(CHEMBL4081876)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Br)c3)ncnc12 |r| Show InChI InChI=1S/C19H20BrN9O3/c1-2-29-26-17(25-27-29)15-13(30)14(31)19(32-15)28-9-24-12-16(22-8-23-18(12)28)21-7-10-4-3-5-11(20)6-10/h3-6,8-9,13-15,19,30-31H,2,7H2,1H3,(H,21,22,23)/t13-,14+,15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human adenosine A2A receptor expressed in CHO cell membranes assessed as stimulation of adenylyl cyclase activity by ... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513538
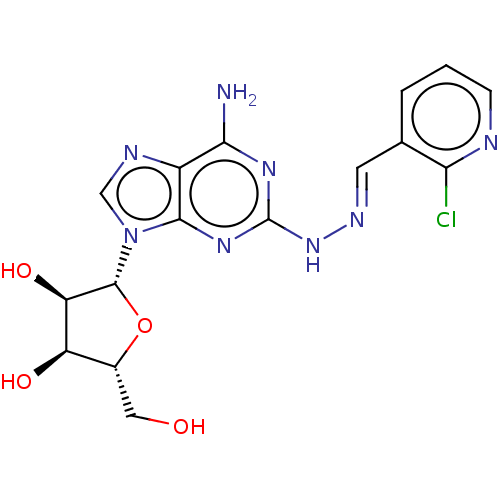
(CHEMBL4454157)Show SMILES Nc1nc(N\N=C\c2cccnc2Cl)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H17ClN8O4/c17-12-7(2-1-3-19-12)4-21-24-16-22-13(18)9-14(23-16)25(6-20-9)15-11(28)10(27)8(5-26)29-15/h1-4,6,8,10-11,15,26-28H,5H2,(H3,18,22,23,24)/b21-4+/t8-,10-,11-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00320
BindingDB Entry DOI: 10.7270/Q2JQ151Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50079321
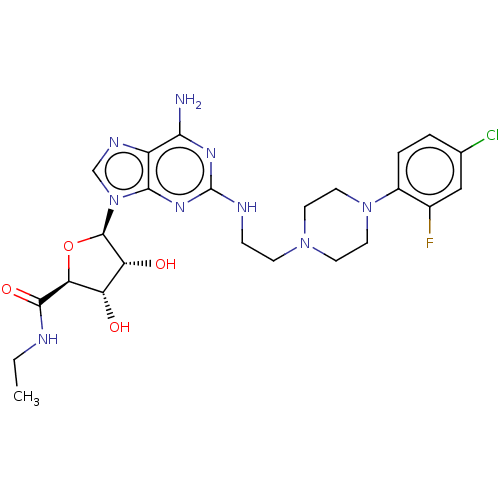
(CHEMBL3416799)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCN3CCN(CC3)c3ccc(Cl)cc3F)nc12 |r| Show InChI InChI=1S/C24H31ClFN9O4/c1-2-28-22(38)19-17(36)18(37)23(39-19)35-12-30-16-20(27)31-24(32-21(16)35)29-5-6-33-7-9-34(10-8-33)15-4-3-13(25)11-14(15)26/h3-4,11-12,17-19,23,36-37H,2,5-10H2,1H3,(H,28,38)(H3,27,29,31,32)/t17-,18+,19-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as stimulation of [3H]cAMP levels by scintillation counting method |
J Med Chem 58: 3253-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00215
BindingDB Entry DOI: 10.7270/Q2X068RF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078427
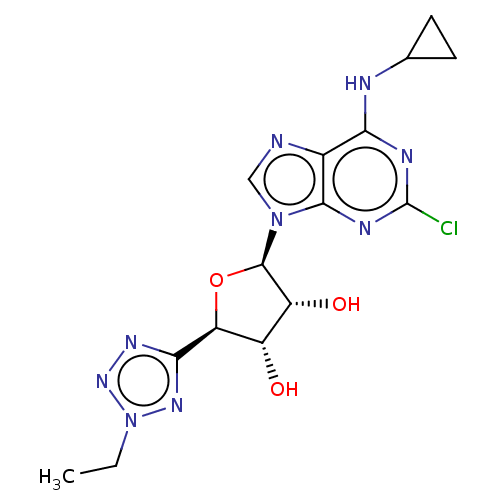
(CHEMBL3414941)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H18ClN9O3/c1-2-25-22-12(21-23-25)10-8(26)9(27)14(28-10)24-5-17-7-11(18-6-3-4-6)19-15(16)20-13(7)24/h5-6,8-10,14,26-27H,2-4H2,1H3,(H,18,19,20)/t8-,9+,10-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513552
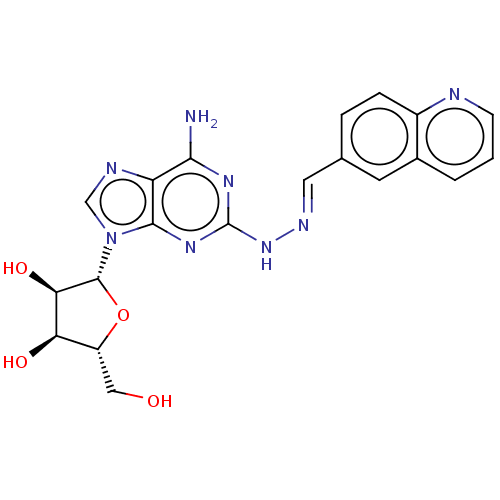
(CHEMBL4458638)Show SMILES Nc1nc(N\N=C\c2ccc3ncccc3c2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H20N8O4/c21-17-14-18(28(9-23-14)19-16(31)15(30)13(8-29)32-19)26-20(25-17)27-24-7-10-3-4-12-11(6-10)2-1-5-22-12/h1-7,9,13,15-16,19,29-31H,8H2,(H3,21,25,26,27)/b24-7+/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50385957
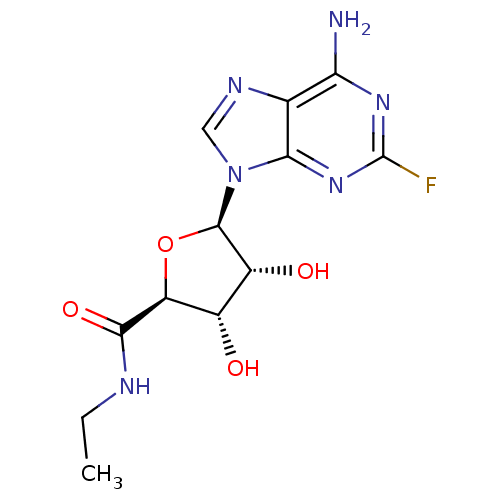
(CHEMBL2042297)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(F)nc12 |r| Show InChI InChI=1S/C12H15FN6O4/c1-2-15-10(22)7-5(20)6(21)11(23-7)19-3-16-4-8(14)17-12(13)18-9(4)19/h3,5-7,11,20-21H,2H2,1H3,(H,15,22)(H2,14,17,18)/t5-,6+,7-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins |
J Med Chem 55: 3521-34 (2012)
Article DOI: 10.1021/jm300206u
BindingDB Entry DOI: 10.7270/Q2FT8N35 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513550
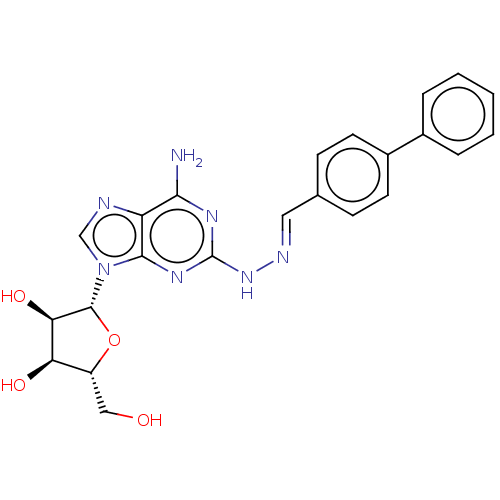
(CHEMBL4564192)Show SMILES Nc1nc(N\N=C\c2ccc(cc2)-c2ccccc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H23N7O4/c24-20-17-21(30(12-25-17)22-19(33)18(32)16(11-31)34-22)28-23(27-20)29-26-10-13-6-8-15(9-7-13)14-4-2-1-3-5-14/h1-10,12,16,18-19,22,31-33H,11H2,(H3,24,27,28,29)/b26-10+/t16-,18-,19-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50026816
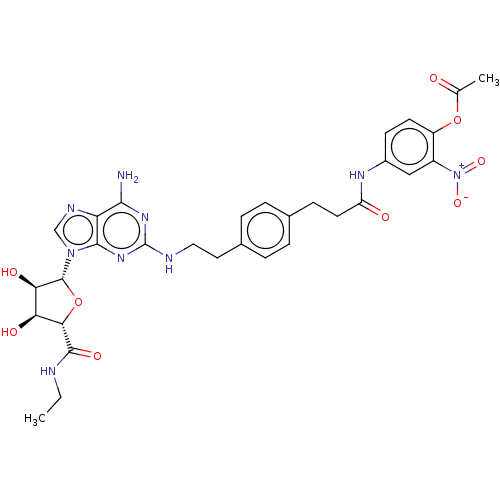
(CHEMBL3335523)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(=O)Nc4ccc(OC(C)=O)c(c4)[N+]([O-])=O)cc3)nc12 |r| Show InChI InChI=1S/C31H35N9O9/c1-3-33-29(45)26-24(43)25(44)30(49-26)39-15-35-23-27(32)37-31(38-28(23)39)34-13-12-18-6-4-17(5-7-18)8-11-22(42)36-19-9-10-21(48-16(2)41)20(14-19)40(46)47/h4-7,9-10,14-15,24-26,30,43-44H,3,8,11-13H2,1-2H3,(H,33,45)(H,36,42)(H3,32,34,37,38)/t24-,25+,26-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2a receptor expressed in CHO cells assessed as stimulation of cAMP formation after 24 hrs |
ACS Med Chem Lett 5: 1043-8 (2014)
Article DOI: 10.1021/ml5002486
BindingDB Entry DOI: 10.7270/Q2VD712G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50385950
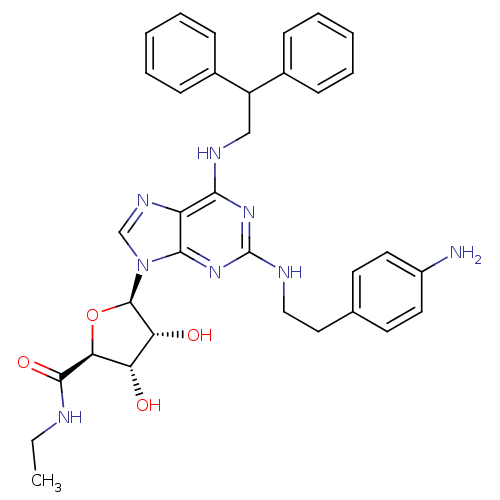
(CHEMBL2042306)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3ccc(N)cc3)nc12 |r| Show InChI InChI=1S/C34H38N8O4/c1-2-36-32(45)29-27(43)28(44)33(46-29)42-20-39-26-30(38-19-25(22-9-5-3-6-10-22)23-11-7-4-8-12-23)40-34(41-31(26)42)37-18-17-21-13-15-24(35)16-14-21/h3-16,20,25,27-29,33,43-44H,2,17-19,35H2,1H3,(H,36,45)(H2,37,38,40,41)/t27-,28+,29-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins |
J Med Chem 55: 3521-34 (2012)
Article DOI: 10.1021/jm300206u
BindingDB Entry DOI: 10.7270/Q2FT8N35 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50385947
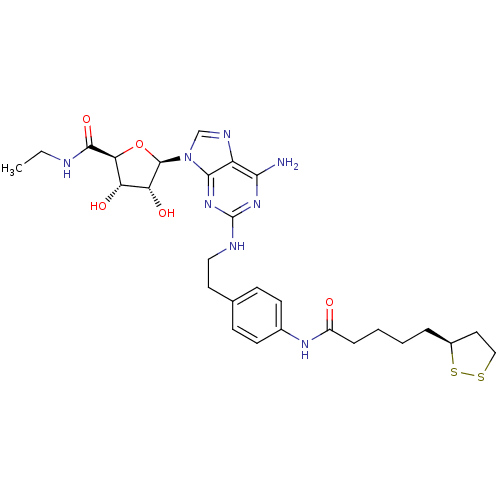
(CHEMBL2042303)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(NC(=O)CCCC[C@H]4CCSS4)cc3)nc12 |r| Show InChI InChI=1S/C28H38N8O5S2/c1-2-30-26(40)23-21(38)22(39)27(41-23)36-15-32-20-24(29)34-28(35-25(20)36)31-13-11-16-7-9-17(10-8-16)33-19(37)6-4-3-5-18-12-14-42-43-18/h7-10,15,18,21-23,27,38-39H,2-6,11-14H2,1H3,(H,30,40)(H,33,37)(H3,29,31,34,35)/t18-,21-,22+,23-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
Monash University
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase in intracellular cAMP level after 30 mins |
J Med Chem 55: 3521-34 (2012)
Article DOI: 10.1021/jm300206u
BindingDB Entry DOI: 10.7270/Q2FT8N35 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266649
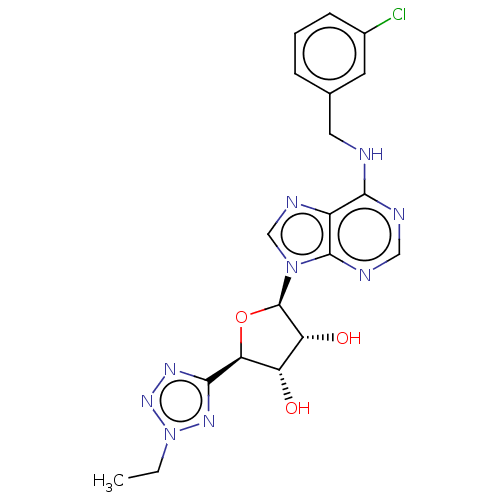
(CHEMBL4071338)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)ncnc12 |r| Show InChI InChI=1S/C19H20ClN9O3/c1-2-29-26-17(25-27-29)15-13(30)14(31)19(32-15)28-9-24-12-16(22-8-23-18(12)28)21-7-10-4-3-5-11(20)6-10/h3-6,8-9,13-15,19,30-31H,2,7H2,1H3,(H,21,22,23)/t13-,14+,15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human adenosine A2A receptor expressed in CHO cell membranes assessed as stimulation of adenylyl cyclase activity by ... |
J Med Chem 60: 4327-4341 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00291
BindingDB Entry DOI: 10.7270/Q2CJ8GZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078428
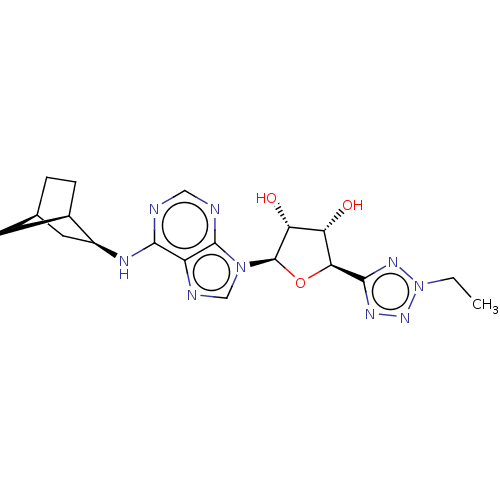
(CHEMBL3414942)Show SMILES [H][C@@]12CC[C@]([H])(C1)[C@H](C2)Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)c1nnn(CC)n1 |r| Show InChI InChI=1S/C19H25N9O3/c1-2-28-25-17(24-26-28)15-13(29)14(30)19(31-15)27-8-22-12-16(20-7-21-18(12)27)23-11-6-9-3-4-10(11)5-9/h7-11,13-15,19,29-30H,2-6H2,1H3,(H,20,21,23)/t9-,10-,11+,13+,14-,15+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150767
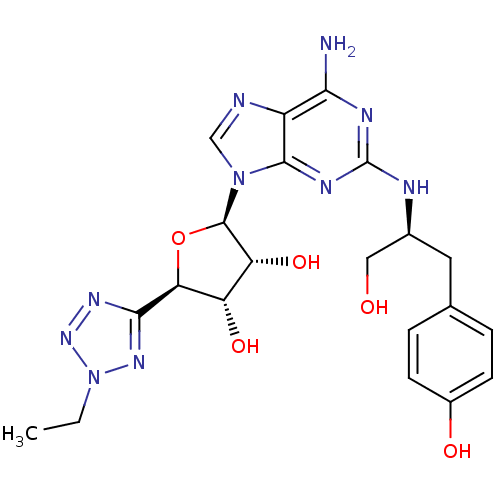
((2R,3R,4S,5R)-2-{6-Amino-2-[(S)-1-hydroxymethyl-2-...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccc(O)cc3)nc12 Show InChI InChI=1S/C21H26N10O5/c1-2-31-28-18(27-29-31)16-14(34)15(35)20(36-16)30-9-23-13-17(22)25-21(26-19(13)30)24-11(8-32)7-10-3-5-12(33)6-4-10/h3-6,9,11,14-16,20,32-35H,2,7-8H2,1H3,(H3,22,24,25,26)/t11-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
IIQAB (CSIC)
Curated by ChEMBL
| Assay Description
Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor |
J Med Chem 47: 4041-53 (2004)
Article DOI: 10.1021/jm031143+
BindingDB Entry DOI: 10.7270/Q26D5TR9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078538
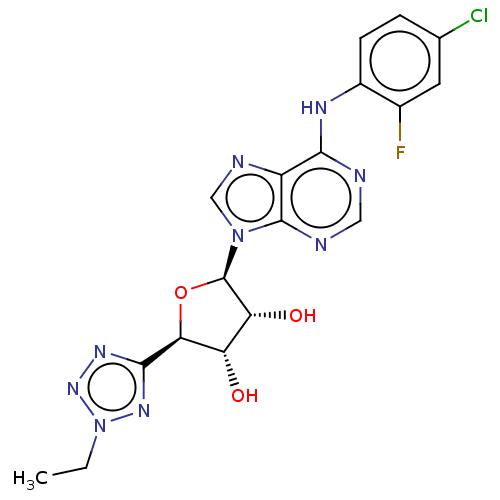
(CHEMBL3414946)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(Nc3ccc(Cl)cc3F)ncnc12 |r| Show InChI InChI=1S/C18H17ClFN9O3/c1-2-29-26-16(25-27-29)14-12(30)13(31)18(32-14)28-7-23-11-15(21-6-22-17(11)28)24-10-4-3-8(19)5-9(10)20/h3-7,12-14,18,30-31H,2H2,1H3,(H,21,22,24)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50513537
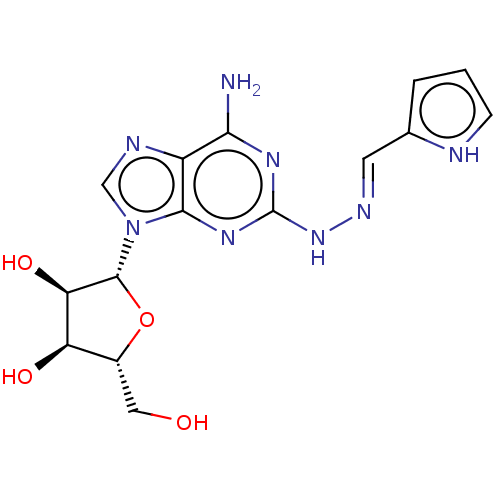
(CHEMBL4468448)Show SMILES Nc1nc(N\N=C\c2ccc[nH]2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H18N8O4/c16-12-9-13(21-15(20-12)22-19-4-7-2-1-3-17-7)23(6-18-9)14-11(26)10(25)8(5-24)27-14/h1-4,6,8,10-11,14,17,24-26H,5H2,(H3,16,20,21,22)/b19-4+/t8-,10-,11-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150766

((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccccc3)nc12 Show InChI InChI=1S/C21H26N10O4/c1-2-31-28-18(27-29-31)16-14(33)15(34)20(35-16)30-10-23-13-17(22)25-21(26-19(13)30)24-12(9-32)8-11-6-4-3-5-7-11/h3-7,10,12,14-16,20,32-34H,2,8-9H2,1H3,(H3,22,24,25,26)/t12-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
IIQAB (CSIC)
Curated by ChEMBL
| Assay Description
Potency against cAMP formation in CHO cells expressing recombinant human A2A receptor |
J Med Chem 47: 4041-53 (2004)
Article DOI: 10.1021/jm031143+
BindingDB Entry DOI: 10.7270/Q26D5TR9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150766

((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccccc3)nc12 Show InChI InChI=1S/C21H26N10O4/c1-2-31-28-18(27-29-31)16-14(33)15(34)20(35-16)30-10-23-13-17(22)25-21(26-19(13)30)24-12(9-32)8-11-6-4-3-5-7-11/h3-7,10,12,14-16,20,32-34H,2,8-9H2,1H3,(H3,22,24,25,26)/t12-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at adenosine receptor A2a (unknown origin) assessed as cAMP formation |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078421
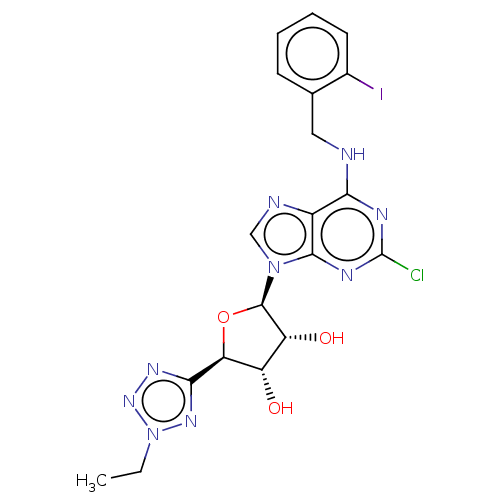
(CHEMBL3414948)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3I)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H19ClIN9O3/c1-2-30-27-16(26-28-30)14-12(31)13(32)18(33-14)29-8-23-11-15(24-19(20)25-17(11)29)22-7-9-5-3-4-6-10(9)21/h3-6,8,12-14,18,31-32H,2,7H2,1H3,(H,22,24,25)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50078426
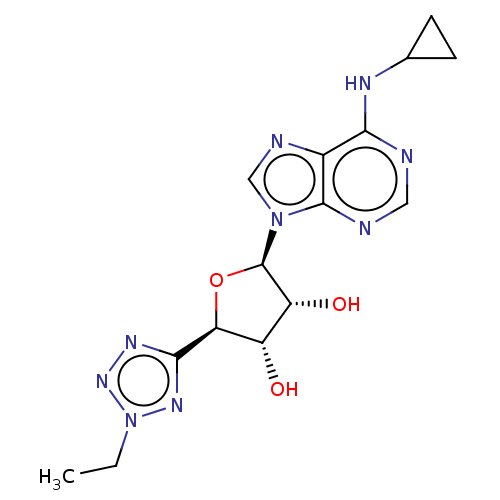
(CHEMBL3414940)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CC3)ncnc12 |r| Show InChI InChI=1S/C15H19N9O3/c1-2-24-21-13(20-22-24)11-9(25)10(26)15(27-11)23-6-18-8-12(19-7-3-4-7)16-5-17-14(8)23/h5-7,9-11,15,25-26H,2-4H2,1H3,(H,16,17,19)/t9-,10+,11-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
University of Camerino
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine A2A receptor transfected in CHO cells assessed as induction of adenylyl cyclase activity |
J Med Chem 58: 2560-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00074
BindingDB Entry DOI: 10.7270/Q2GX4D8B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150077
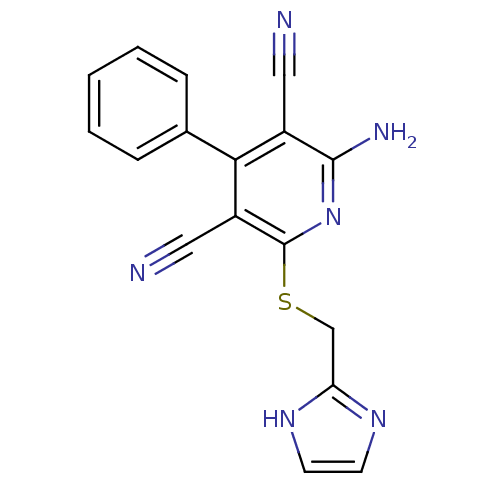
(2-((1H-imidazol-2-yl)methylthio)-6-amino-4-phenylp...)Show InChI InChI=1S/C17H12N6S/c18-8-12-15(11-4-2-1-3-5-11)13(9-19)17(23-16(12)20)24-10-14-21-6-7-22-14/h1-7H,10H2,(H2,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Partial agonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as intrinsic activity by measuring cell index change withi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01856
BindingDB Entry DOI: 10.7270/Q2PN99H7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
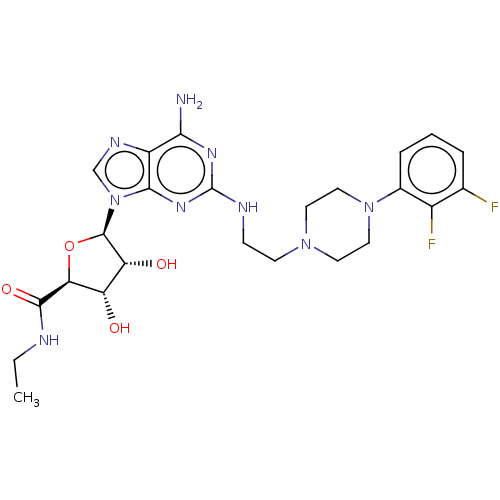
(Homo sapiens (Human)) | BDBM50079322
(CHEMBL3416798)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCN3CCN(CC3)c3cccc(F)c3F)nc12 |r| Show InChI InChI=1S/C24H31F2N9O4/c1-2-28-22(38)19-17(36)18(37)23(39-19)35-12-30-16-20(27)31-24(32-21(16)35)29-6-7-33-8-10-34(11-9-33)14-5-3-4-13(25)15(14)26/h3-5,12,17-19,23,36-37H,2,6-11H2,1H3,(H,28,38)(H3,27,29,31,32)/t17-,18+,19-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as stimulation of [3H]cAMP levels by scintillation counting method |
J Med Chem 58: 3253-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00215
BindingDB Entry DOI: 10.7270/Q2X068RF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
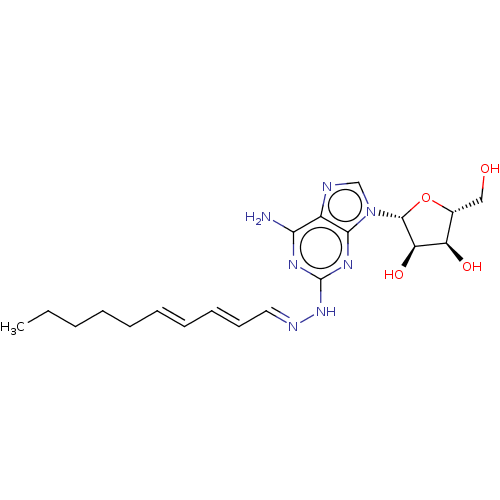
(Homo sapiens (Human)) | BDBM50513531
(CHEMBL4435740)Show SMILES CCCCC\C=C\C=C\C=N\Nc1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C20H29N7O4/c1-2-3-4-5-6-7-8-9-10-23-26-20-24-17(21)14-18(25-20)27(12-22-14)19-16(30)15(29)13(11-28)31-19/h6-10,12-13,15-16,19,28-30H,2-5,11H2,1H3,(H3,21,24,25,26)/b7-6+,9-8+,23-10+/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Agonist activity at human adenosine receptor A2A expressed in HEK293 cells assessed as cAMP accumulation incubated for 30 mins by Eu-cAMP tracer-base... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050
BindingDB Entry DOI: 10.7270/Q24B34NM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data