 Found 5151 hits of ec50 data for polymerid = 2137,49000332,6516
Found 5151 hits of ec50 data for polymerid = 2137,49000332,6516 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213614
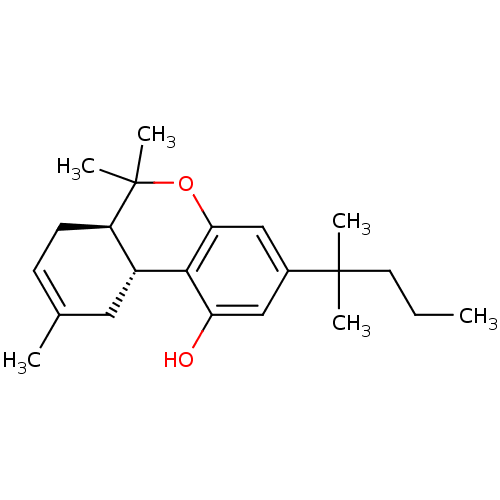
((6aR,10aR)-6,6,9-trimethyl-3-(2-methylpentan-2-yl)...)Show SMILES CCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:14| Show InChI InChI=1S/C22H32O2/c1-7-10-21(3,4)15-12-18(23)20-16-11-14(2)8-9-17(16)22(5,6)24-19(20)13-15/h8,12-13,16-17,23H,7,9-11H2,1-6H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213614

((6aR,10aR)-6,6,9-trimethyl-3-(2-methylpentan-2-yl)...)Show SMILES CCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:14| Show InChI InChI=1S/C22H32O2/c1-7-10-21(3,4)15-12-18(23)20-16-11-14(2)8-9-17(16)22(5,6)24-19(20)13-15/h8,12-13,16-17,23H,7,9-11H2,1-6H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213614

((6aR,10aR)-6,6,9-trimethyl-3-(2-methylpentan-2-yl)...)Show SMILES CCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:14| Show InChI InChI=1S/C22H32O2/c1-7-10-21(3,4)15-12-18(23)20-16-11-14(2)8-9-17(16)22(5,6)24-19(20)13-15/h8,12-13,16-17,23H,7,9-11H2,1-6H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50213614

((6aR,10aR)-6,6,9-trimethyl-3-(2-methylpentan-2-yl)...)Show SMILES CCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:14| Show InChI InChI=1S/C22H32O2/c1-7-10-21(3,4)15-12-18(23)20-16-11-14(2)8-9-17(16)22(5,6)24-19(20)13-15/h8,12-13,16-17,23H,7,9-11H2,1-6H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00561
BindingDB Entry DOI: 10.7270/Q2KS6WG4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272395
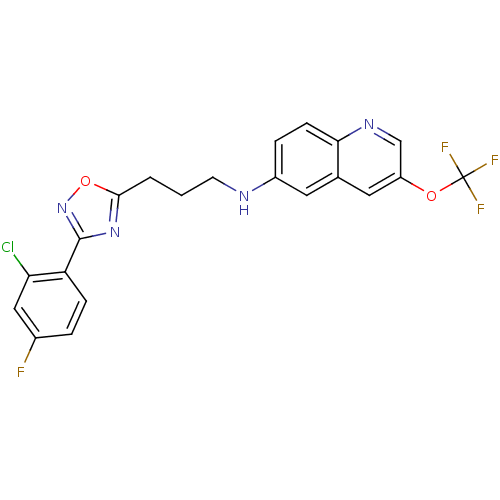
(CHEMBL499066 | N-(3-(3-(2-Chloro-4-fluorophenyl)-1...)Show SMILES Fc1ccc(-c2noc(CCCNc3ccc4ncc(OC(F)(F)F)cc4c3)n2)c(Cl)c1 Show InChI InChI=1S/C21H15ClF4N4O2/c22-17-10-13(23)3-5-16(17)20-29-19(32-30-20)2-1-7-27-14-4-6-18-12(8-14)9-15(11-28-18)31-21(24,25)26/h3-6,8-11,27H,1-2,7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assay |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50063927
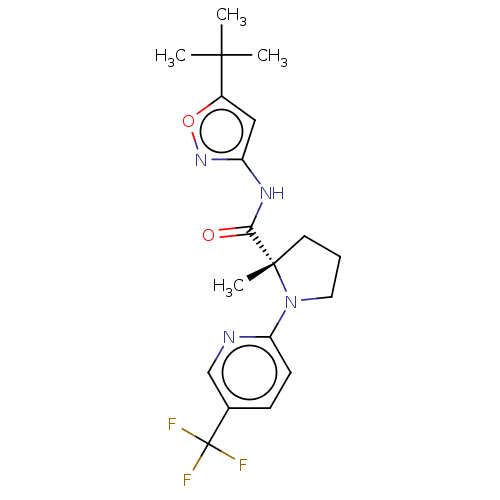
(CHEMBL3400946)Show SMILES CC(C)(C)c1cc(NC(=O)[C@]2(C)CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-17(2,3)13-10-14(25-28-13)24-16(27)18(4)8-5-9-26(18)15-7-6-12(11-23-15)19(20,21)22/h6-7,10-11H,5,8-9H2,1-4H3,(H,24,25,27)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006258

(CHEMBL3234701)Show SMILES CC(C)CCS(=O)(=O)C(C)(C)C(=O)Nc1cc(no1)C(C)(C)C Show InChI InChI=1S/C16H28N2O4S/c1-11(2)8-9-23(20,21)16(6,7)14(19)17-13-10-12(18-22-13)15(3,4)5/h10-11H,8-9H2,1-7H3,(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor assessed as inhibition of forskolin-induced cAMP production preincubated for 15 mins followed by forskolin cha... |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 90 mins by liquid scintillation counting |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3... |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50303551
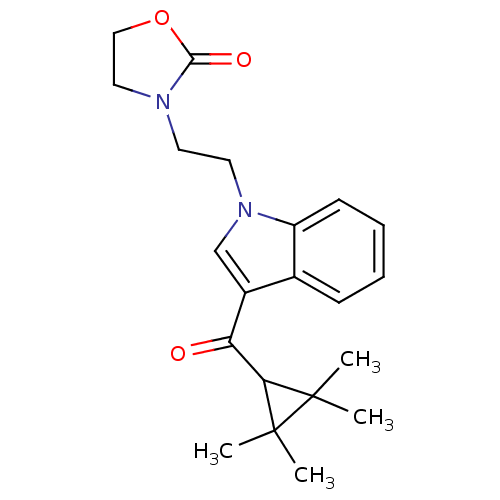
(3-(2-(3-(2,2,3,3-Tetramethylcyclopropanecarbonyl)-...)Show SMILES CC1(C)C(C(=O)c2cn(CCN3CCOC3=O)c3ccccc23)C1(C)C Show InChI InChI=1S/C21H26N2O3/c1-20(2)18(21(20,3)4)17(24)15-13-23(16-8-6-5-7-14(15)16)10-9-22-11-12-26-19(22)25/h5-8,13,18H,9-12H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at rat CB2 receptor assessed as inhibition of forskolin-induced cAMP production by cell based assay |
J Med Chem 53: 295-315 (2010)
Article DOI: 10.1021/jm901214q
BindingDB Entry DOI: 10.7270/Q2KD1Z00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006259

(CHEMBL3234706)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-17(2,3)13-9-14(24-27-13)23-16(26)12-5-4-8-25(12)15-7-6-11(10-22-15)18(19,20)21/h6-7,9-10,12H,4-5,8H2,1-3H3,(H,23,24,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50000727
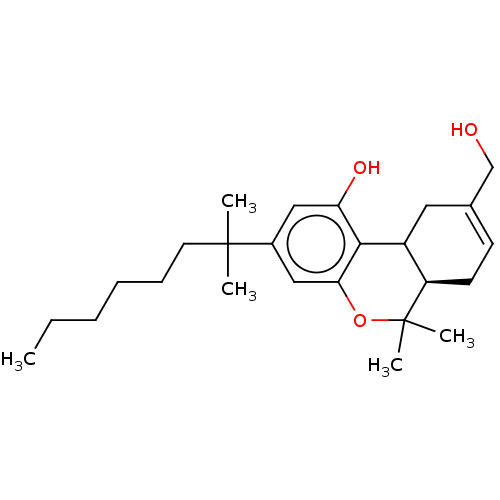
((R)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-di...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2C3CC(CO)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19?,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a |
Institute of Science
Curated by ChEMBL
| Assay Description
Effective concentration for inhibition of human Cannabinoid receptor 2-mediated adenylylcyclase using African green monkey (COS-7) cells transfected ... |
J Med Chem 40: 3228-33 (1997)
Article DOI: 10.1021/jm970126f
BindingDB Entry DOI: 10.7270/Q27943TD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50063924
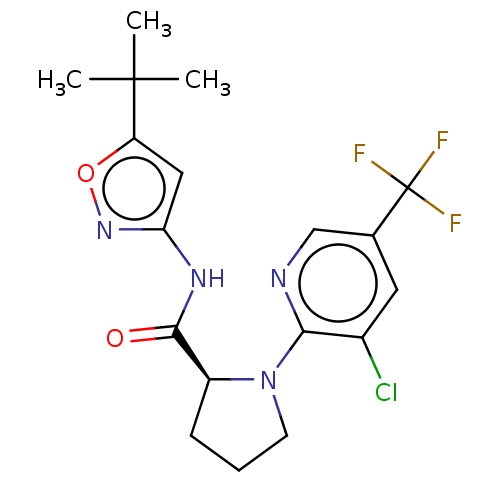
(CHEMBL3400943)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ncc(cc2Cl)C(F)(F)F)no1 |r| Show InChI InChI=1S/C18H20ClF3N4O2/c1-17(2,3)13-8-14(25-28-13)24-16(27)12-5-4-6-26(12)15-11(19)7-10(9-23-15)18(20,21)22/h7-9,12H,4-6H2,1-3H3,(H,24,25,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50288371
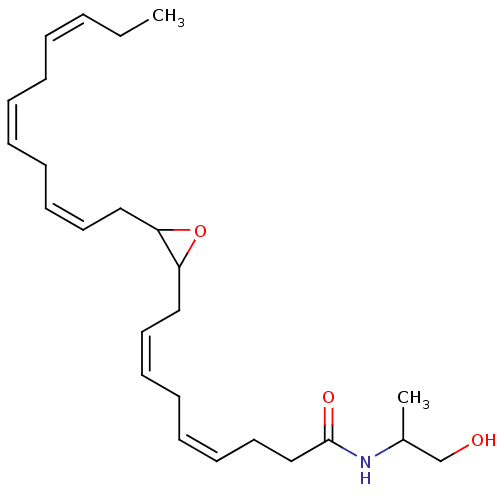
(CHEMBL4177060 | US11472787, Compound 10,11-EDP-IA)Show SMILES CC\C=C/C\C=C/C\C=C/CC1OC1C\C=C/C\C=C/CCC(=O)NC(C)CO Show InChI InChI=1S/C25H39NO3/c1-3-4-5-6-7-8-9-12-15-18-23-24(29-23)19-16-13-10-11-14-17-20-25(28)26-22(2)21-27/h4-5,7-8,11-16,22-24,27H,3,6,9-10,17-21H2,1-2H3,(H,26,28)/b5-4-,8-7-,14-11-,15-12-,16-13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QC06SQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50288371

(CHEMBL4177060 | US11472787, Compound 10,11-EDP-IA)Show SMILES CC\C=C/C\C=C/C\C=C/CC1OC1C\C=C/C\C=C/CCC(=O)NC(C)CO Show InChI InChI=1S/C25H39NO3/c1-3-4-5-6-7-8-9-12-15-18-23-24(29-23)19-16-13-10-11-14-17-20-25(28)26-22(2)21-27/h4-5,7-8,11-16,22-24,27H,3,6,9-10,17-21H2,1-2H3,(H,26,28)/b5-4-,8-7-,14-11-,15-12-,16-13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at N-terminal FLAG-tagged human CB2 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte... |
J Med Chem 61: 5569-5579 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00243
BindingDB Entry DOI: 10.7270/Q2P84FDV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272233
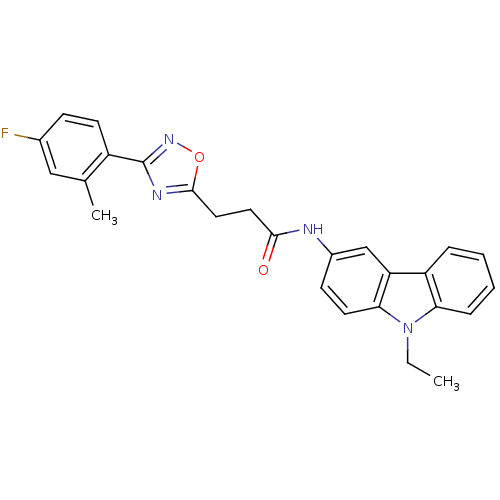
(CHEMBL501472 | N-(9-Ethyl-9H-carbazol-3-yl)-3-(3-(...)Show SMILES CCn1c2ccccc2c2cc(NC(=O)CCc3nc(no3)-c3ccc(F)cc3C)ccc12 Show InChI InChI=1S/C26H23FN4O2/c1-3-31-22-7-5-4-6-20(22)21-15-18(9-11-23(21)31)28-24(32)12-13-25-29-26(30-33-25)19-10-8-17(27)14-16(19)2/h4-11,14-15H,3,12-13H2,1-2H3,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assay |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006233
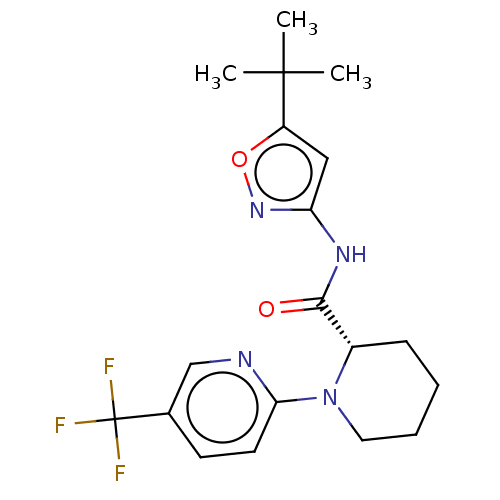
(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006233

(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3... |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006233

(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 90 mins by liquid scintillation counting |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006233

(CHEMBL3235059)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2c2ccc(cn2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H23F3N4O2/c1-18(2,3)14-10-15(25-28-14)24-17(27)13-6-4-5-9-26(13)16-8-7-12(11-23-16)19(20,21)22/h7-8,10-11,13H,4-6,9H2,1-3H3,(H,24,25,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM210526

(US9290451, 233)Show SMILES CCC(CC)(NC(=O)c1ccc(C2CC2)c(OCC2CCCO2)n1)C(=O)OC Show InChI InChI=1S/C21H30N2O5/c1-4-21(5-2,20(25)26-3)23-18(24)17-11-10-16(14-8-9-14)19(22-17)28-13-15-7-6-12-27-15/h10-11,14-15H,4-9,12-13H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9290451 (2016)
BindingDB Entry DOI: 10.7270/Q20Z724J |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM260128
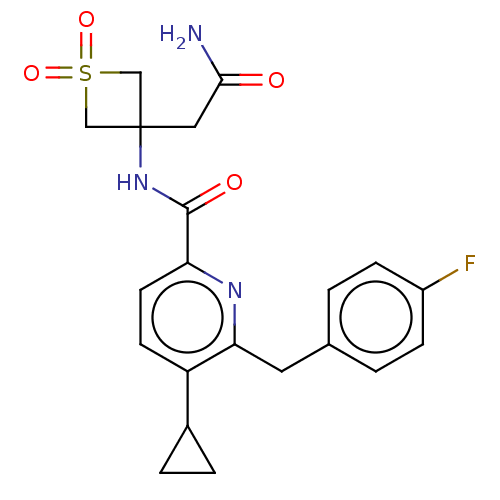
(US9522886, 76 | US9522886, 81)Show SMILES NC(=O)CC1(CS(=O)(=O)C1)NC(=O)c1ccc(C2CC2)c(Cc2ccc(F)cc2)n1 Show InChI InChI=1S/C21H22FN3O4S/c22-15-5-1-13(2-6-15)9-18-16(14-3-4-14)7-8-17(24-18)20(27)25-21(10-19(23)26)11-30(28,29)12-21/h1-2,5-8,14H,3-4,9-12H2,(H2,23,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9522886 (2016)
BindingDB Entry DOI: 10.7270/Q28W3C7W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50272234
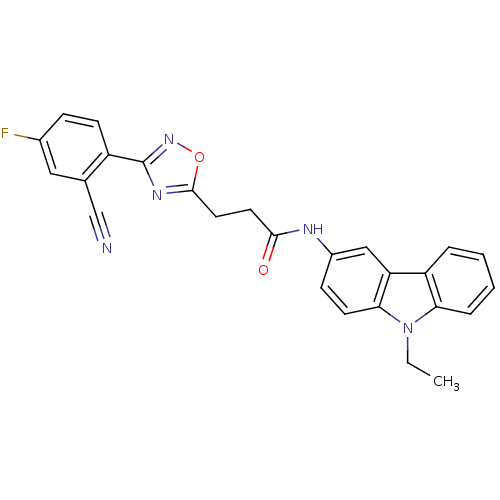
(3-(3-(2-Cyano-4-fluorophenyl)-1,2,4-oxadiazol-5-yl...)Show SMILES CCn1c2ccccc2c2cc(NC(=O)CCc3nc(no3)-c3ccc(F)cc3C#N)ccc12 Show InChI InChI=1S/C26H20FN5O2/c1-2-32-22-6-4-3-5-20(22)21-14-18(8-10-23(21)32)29-24(33)11-12-25-30-26(31-34-25)19-9-7-17(27)13-16(19)15-28/h3-10,13-14H,2,11-12H2,1H3,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at rat recombinant CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated intracellular cAMP level |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50006224

(CHEMBL3234674)Show SMILES CC(C)(C)Cc1nc2cc(ccc2n1CC1CC1)S(=O)(=O)C1(CC1)C(N)=O Show InChI InChI=1S/C20H27N3O3S/c1-19(2,3)11-17-22-15-10-14(27(25,26)20(8-9-20)18(21)24)6-7-16(15)23(17)12-13-4-5-13/h6-7,10,13H,4-5,8-9,11-12H2,1-3H3,(H2,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor transfected in CHO-K1 cells assessed as cAMP change |
Cell Chem Biol 56: 8224-56 (2013)
Article DOI: 10.1021/jm4005626
BindingDB Entry DOI: 10.7270/Q2B859M5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM21284
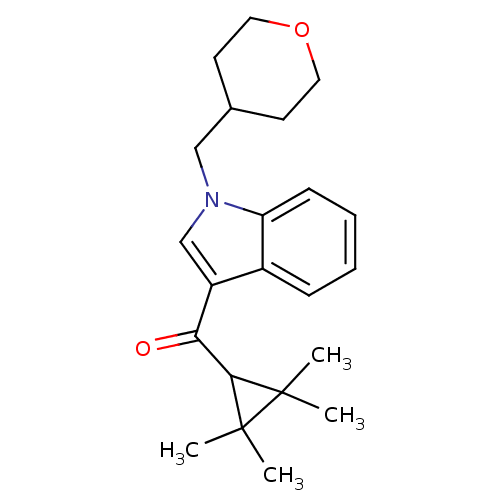
(1-(oxan-4-ylmethyl)-3-[(2,2,3,3-tetramethylcyclopr...)Show SMILES CC1(C)C(C(=O)c2cn(CC3CCOCC3)c3ccccc23)C1(C)C Show InChI InChI=1S/C22H29NO2/c1-21(2)20(22(21,3)4)19(24)17-14-23(13-15-9-11-25-12-10-15)18-8-6-5-7-16(17)18/h5-8,14-15,20H,9-13H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at rat CB2 receptor assessed as inhibition of forskolin-induced cAMP production by cell based assay |
J Med Chem 53: 295-315 (2010)
Article DOI: 10.1021/jm901214q
BindingDB Entry DOI: 10.7270/Q2KD1Z00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042590
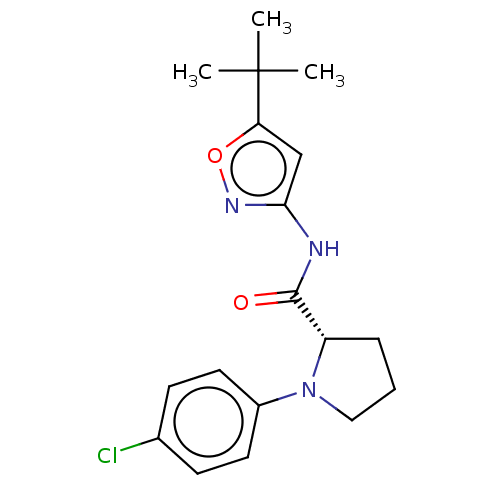
(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042704
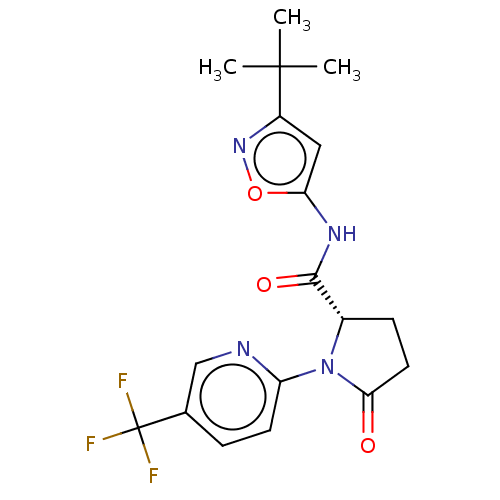
(CHEMBL3353885)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCC(=O)N2c2ccc(cn2)C(F)(F)F)on1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-17(2,3)12-8-14(28-24-12)23-16(27)11-5-7-15(26)25(11)13-6-4-10(9-22-13)18(19,20)21/h4,6,8-9,11H,5,7H2,1-3H3,(H,23,27)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272346
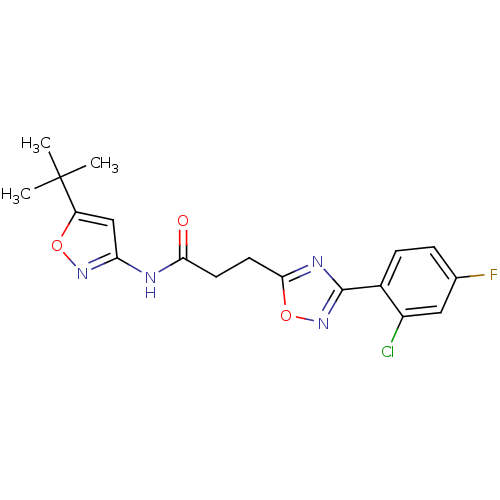
(CHEMBL499060 | N-(5-tert-Butylisoxazol-3-yl)-3-(3-...)Show SMILES CC(C)(C)c1cc(NC(=O)CCc2nc(no2)-c2ccc(F)cc2Cl)no1 Show InChI InChI=1S/C18H18ClFN4O3/c1-18(2,3)13-9-14(23-26-13)21-15(25)6-7-16-22-17(24-27-16)11-5-4-10(20)8-12(11)19/h4-5,8-9H,6-7H2,1-3H3,(H,21,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in Sf9 cells by GTP-europium binding assay |
J Med Chem 51: 5019-34 (2008)
Article DOI: 10.1021/jm800463f
BindingDB Entry DOI: 10.7270/Q2W096VS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042590

(CHEMBL3353863)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C18H22ClN3O2/c1-18(2,3)15-11-16(21-24-15)20-17(23)14-5-4-10-22(14)13-8-6-12(19)7-9-13/h6-9,11,14H,4-5,10H2,1-3H3,(H,20,21,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3... |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042891
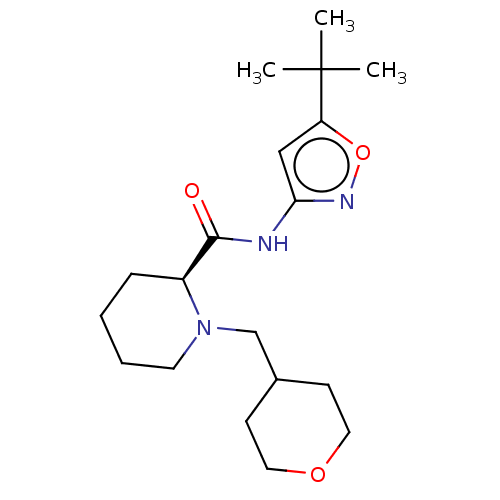
(CHEMBL3354535)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCCN2CC2CCOCC2)no1 |r| Show InChI InChI=1S/C19H31N3O3/c1-19(2,3)16-12-17(21-25-16)20-18(23)15-6-4-5-9-22(15)13-14-7-10-24-11-8-14/h12,14-15H,4-11,13H2,1-3H3,(H,20,21,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressing CHO cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for 3... |
Bioorg Med Chem Lett 25: 587-92 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.031
BindingDB Entry DOI: 10.7270/Q2QZ2CM5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50041002
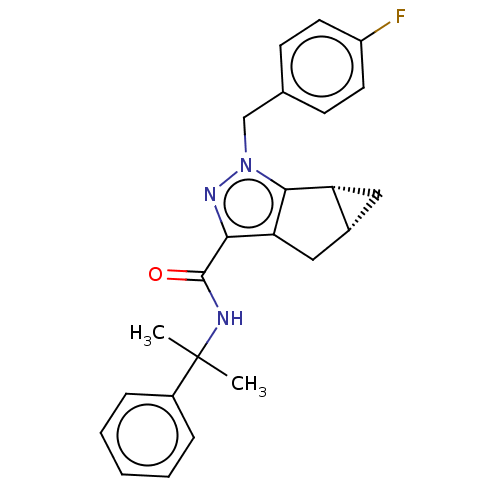
(CHEMBL3354941)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)c(nn1Cc1ccc(F)cc1)C(=O)NC(C)(C)c1ccccc1 |r| Show InChI InChI=1S/C24H24FN3O/c1-24(2,17-6-4-3-5-7-17)26-23(29)21-20-13-16-12-19(16)22(20)28(27-21)14-15-8-10-18(25)11-9-15/h3-11,16,19H,12-14H2,1-2H3,(H,26,29)/t16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant CB2 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method |
Bioorg Med Chem Lett 25: 322-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.040
BindingDB Entry DOI: 10.7270/Q21J9CDS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042585
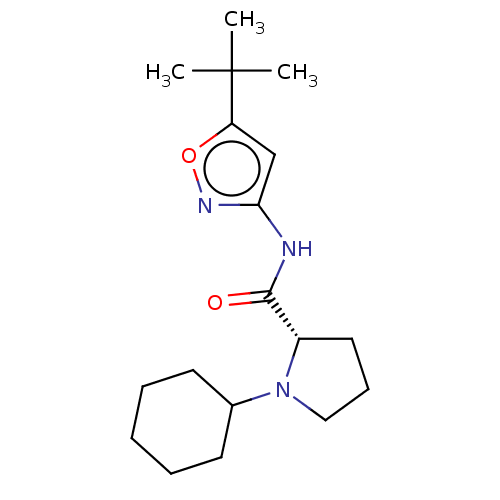
(CHEMBL3353864)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2C2CCCCC2)no1 |r| Show InChI InChI=1S/C18H29N3O2/c1-18(2,3)15-12-16(20-23-15)19-17(22)14-10-7-11-21(14)13-8-5-4-6-9-13/h12-14H,4-11H2,1-3H3,(H,19,20,22)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM210658
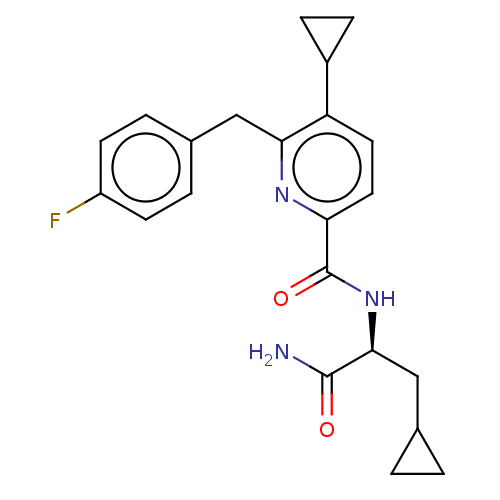
(US9290451, 326)Show SMILES NC(=O)[C@H](CC1CC1)NC(=O)c1ccc(C2CC2)c(Cc2ccc(F)cc2)n1 |r| Show InChI InChI=1S/C22H24FN3O2/c23-16-7-3-14(4-8-16)11-19-17(15-5-6-15)9-10-18(25-19)22(28)26-20(21(24)27)12-13-1-2-13/h3-4,7-10,13,15,20H,1-2,5-6,11-12H2,(H2,24,27)(H,26,28)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9290451 (2016)
BindingDB Entry DOI: 10.7270/Q20Z724J |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM221150

(US9303012, 76)Show SMILES NC(=O)[C@@H]1CC(F)(F)CN1C(=O)c1ccc(C2CC2)c(Cc2ccc(F)cc2)n1 |r| Show InChI InChI=1S/C21H20F3N3O2/c22-14-5-1-12(2-6-14)9-17-15(13-3-4-13)7-8-16(26-17)20(29)27-11-21(23,24)10-18(27)19(25)28/h1-2,5-8,13,18H,3-4,9-11H2,(H2,25,28)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | 37 |
HOFFMAN-LA ROCHE INC.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9303012 (2016)
BindingDB Entry DOI: 10.7270/Q2FF3R7H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50061108
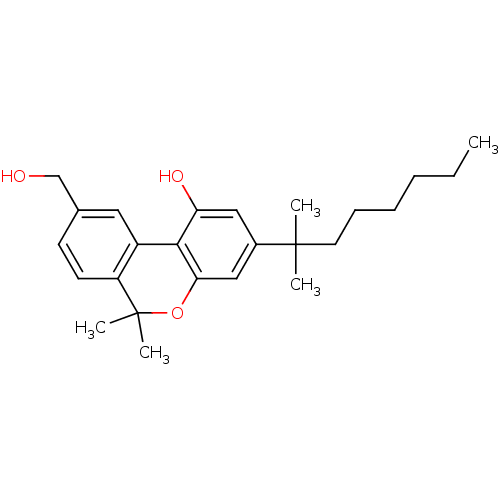
(3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-dimeth...)Show SMILES CCCCCCC(C)(C)c1cc(O)c-2c(OC(C)(C)c3ccc(CO)cc-23)c1 Show InChI InChI=1S/C25H34O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10-11,13-15,26-27H,6-9,12,16H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.208 | n/a | n/a | n/a | n/a |
Institute of Science
Curated by ChEMBL
| Assay Description
Effective concentration for inhibition of human Cannabinoid receptor 2-mediated adenylylcyclase using African green monkey (COS-7) cells transfected ... |
J Med Chem 40: 3228-33 (1997)
Article DOI: 10.1021/jm970126f
BindingDB Entry DOI: 10.7270/Q27943TD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50063926
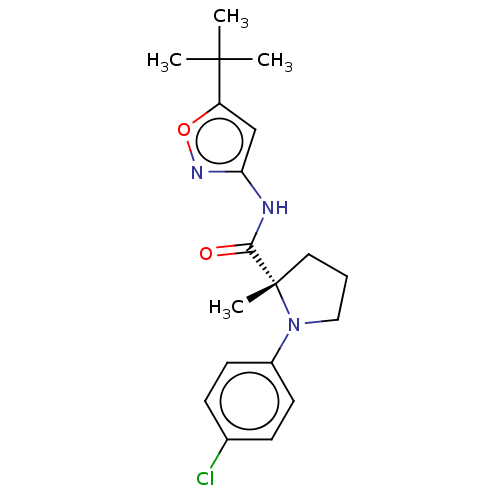
(CHEMBL3400945)Show SMILES CC(C)(C)c1cc(NC(=O)[C@]2(C)CCCN2c2ccc(Cl)cc2)no1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-18(2,3)15-12-16(22-25-15)21-17(24)19(4)10-5-11-23(19)14-8-6-13(20)7-9-14/h6-9,12H,5,10-11H2,1-4H3,(H,21,22,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM295696
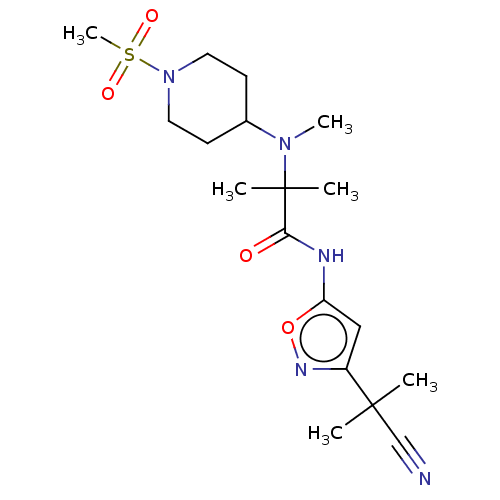
(US10112934, Example 5 | US10570125, Example 5 | US...)Show SMILES CN(C1CCN(CC1)S(C)(=O)=O)C(C)(C)C(=O)Nc1cc(no1)C(C)(C)C#N Show InChI InChI=1S/C18H29N5O4S/c1-17(2,12-19)14-11-15(27-21-14)20-16(24)18(3,4)22(5)13-7-9-23(10-8-13)28(6,25)26/h11,13H,7-10H2,1-6H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37° C. A... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q25Q508W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM295696

(US10112934, Example 5 | US10570125, Example 5 | US...)Show SMILES CN(C1CCN(CC1)S(C)(=O)=O)C(C)(C)C(=O)Nc1cc(no1)C(C)(C)C#N Show InChI InChI=1S/C18H29N5O4S/c1-17(2,12-19)14-11-15(27-21-14)20-16(24)18(3,4)22(5)13-7-9-23(10-8-13)28(6,25)26/h11,13H,7-10H2,1-6H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2VT1X7B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins |
Bioorg Med Chem Lett 25: 575-80 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.033
BindingDB Entry DOI: 10.7270/Q2222WGQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM295696

(US10112934, Example 5 | US10570125, Example 5 | US...)Show SMILES CN(C1CCN(CC1)S(C)(=O)=O)C(C)(C)C(=O)Nc1cc(no1)C(C)(C)C#N Show InChI InChI=1S/C18H29N5O4S/c1-17(2,12-19)14-11-15(27-21-14)20-16(24)18(3,4)22(5)13-7-9-23(10-8-13)28(6,25)26/h11,13H,7-10H2,1-6H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation
US Patent
| Assay Description
CB2: CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37°... |
US Patent US10570125 (2020)
BindingDB Entry DOI: 10.7270/Q21C209H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50042591

(CHEMBL3353862)Show SMILES CC(C)(C)c1cc(NC(=O)[C@@H]2CCCN2c2ccc(cc2)C(F)(F)F)no1 |r| Show InChI InChI=1S/C19H22F3N3O2/c1-18(2,3)15-11-16(24-27-15)23-17(26)14-5-4-10-25(14)13-8-6-12(7-9-13)19(20,21)22/h6-9,11,14H,4-5,10H2,1-3H3,(H,23,24,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins |
Bioorg Med Chem Lett 25: 581-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.019
BindingDB Entry DOI: 10.7270/Q2CN75H1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM295696

(US10112934, Example 5 | US10570125, Example 5 | US...)Show SMILES CN(C1CCN(CC1)S(C)(=O)=O)C(C)(C)C(=O)Nc1cc(no1)C(C)(C)C#N Show InChI InChI=1S/C18H29N5O4S/c1-17(2,12-19)14-11-15(27-21-14)20-16(24)18(3,4)22(5)13-7-9-23(10-8-13)28(6,25)26/h11,13H,7-10H2,1-6H3,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Centrexion Therapeutics Corporation
US Patent
| Assay Description
CHO cells expressing human CB2R (Euroscreen) were plated at a density of 10,000 cells per well in 384 well plates and incubated overnight at 37° C. A... |
US Patent US10112934 (2018)
BindingDB Entry DOI: 10.7270/Q2F191RP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM240353
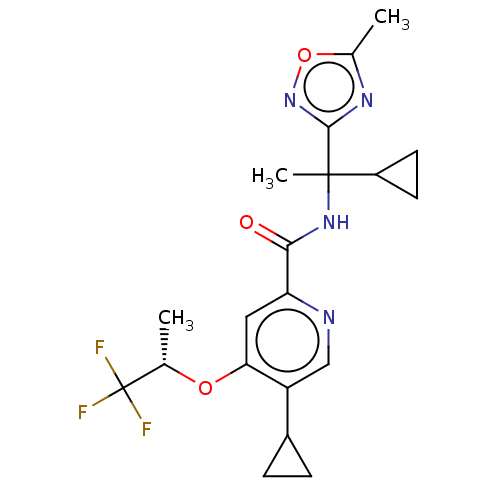
(US9409866, 68 | US9409866, 69)Show SMILES C[C@H](Oc1cc(ncc1C1CC1)C(=O)NC(C)(C1CC1)c1noc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N4O3/c1-10(20(21,22)23)29-16-8-15(24-9-14(16)12-4-5-12)17(28)26-19(3,13-6-7-13)18-25-11(2)30-27-18/h8-10,12-13H,4-7H2,1-3H3,(H,26,28)/t10-,19?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9409866 (2016)
BindingDB Entry DOI: 10.7270/Q2FX78BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM240357
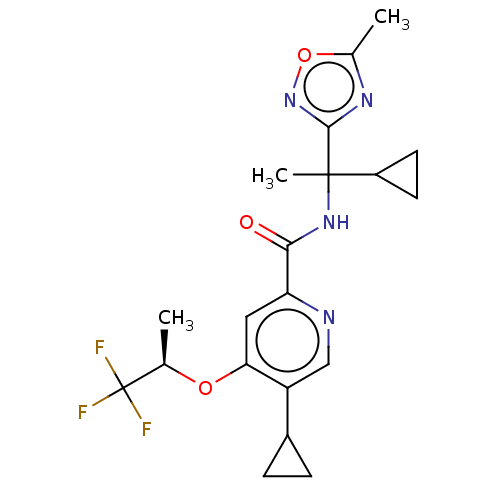
(US9409866, 72 | US9409866, 73)Show SMILES C[C@@H](Oc1cc(ncc1C1CC1)C(=O)NC(C)(C1CC1)c1noc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N4O3/c1-10(20(21,22)23)29-16-8-15(24-9-14(16)12-4-5-12)17(28)26-19(3,13-6-7-13)18-25-11(2)30-27-18/h8-10,12-13H,4-7H2,1-3H3,(H,26,28)/t10-,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | 37 |
HOFFMANN-LA ROCHE INC.
US Patent
| Assay Description
CHO cells expressing human CB1 or CB2 receptors are seeded 17-24 hours prior to the experiment 50.000 cells per well in a black 96 well plate with fl... |
US Patent US9409866 (2016)
BindingDB Entry DOI: 10.7270/Q2FX78BD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067498
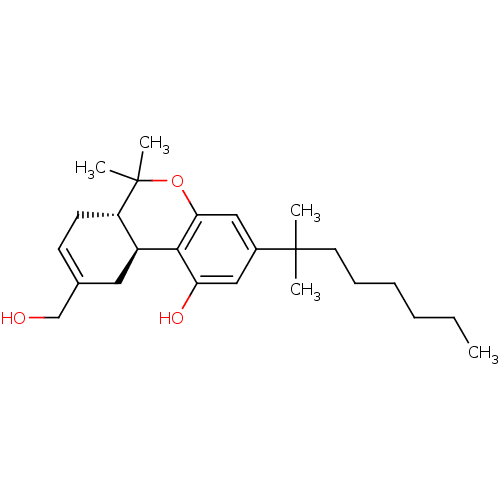
((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@H]3CC(CO)=CC[C@@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H38O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-15,19-20,26-27H,6-9,11-13,16H2,1-5H3/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
University of Kuopio
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor transfected in CHO cells by [35]GTPgamma binding assay |
J Med Chem 48: 7166-71 (2005)
Article DOI: 10.1021/jm050565b
BindingDB Entry DOI: 10.7270/Q2PC3552 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data