 Found 3773 hits of ec50 data for polymerid = 2179,50003700
Found 3773 hits of ec50 data for polymerid = 2179,50003700 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM465458
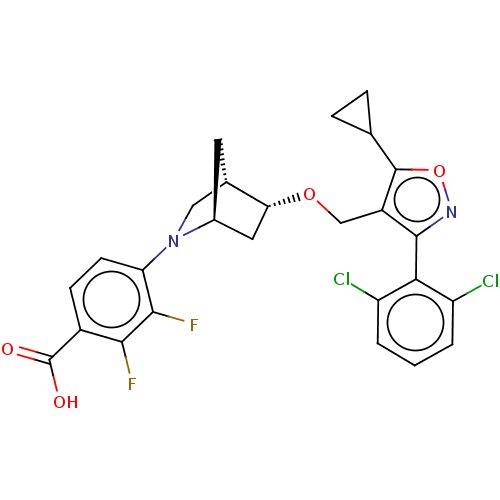
(US10793568, Compound I-110)Show SMILES OC(=O)c1ccc(N2C[C@@H]3C[C@H]2C[C@H]3OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)c(F)c1F |wU:13.15,9.9,wD:11.10,THB:14:13:8.7:10,6:7:13.12:10,(4.05,-10.8,;5.06,-9.63,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.26,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,;5.07,-5.56,;6.09,-4.4,;5.57,-7.02,;7.09,-7.31,)| Show InChI InChI=1S/C26H22Cl2F2N2O4/c27-17-2-1-3-18(28)21(17)24-16(25(36-31-24)12-4-5-12)11-35-20-9-14-8-13(20)10-32(14)19-7-6-15(26(33)34)22(29)23(19)30/h1-3,6-7,12-14,20H,4-5,8-11H2,(H,33,34)/t13-,14-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465567

(US10793568, Compound I-217)Show SMILES CC(O)CCS(=O)(=O)NC(=O)c1ccc(cc1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:23.26,19.20,wD:21.21,THB:24:23:18.17:20,14:17:23.22:20,(11.09,-14.89,;9.58,-14.6,;8.57,-15.76,;9.08,-13.14,;7.57,-12.84,;7.07,-11.39,;5.62,-11.89,;8.53,-10.89,;6.57,-9.93,;5.06,-9.63,;4.05,-10.8,;4.56,-8.18,;5.57,-7.02,;5.07,-5.56,;3.56,-5.26,;2.55,-6.43,;3.05,-7.88,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.84,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.35,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C30H33Cl2N3O6S/c1-17(36)11-12-42(38,39)34-30(37)19-7-9-21(10-8-19)35-15-20-13-22(35)14-26(20)40-16-23-28(33-41-29(23)18-5-6-18)27-24(31)3-2-4-25(27)32/h2-4,7-10,17-18,20,22,26,36H,5-6,11-16H2,1H3,(H,34,37)/t17?,20-,22-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465447
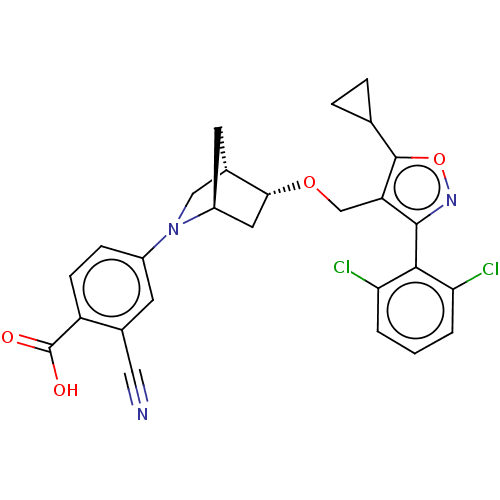
(US10793568, Compound I-101)Show SMILES OC(=O)c1ccc(cc1C#N)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:15.15,17.20,wD:13.14,TLB:18:17:11.12:14,6:11:16.17:14,(-3.33,3.01,;-3.65,1.5,;-5.11,1.02,;-2.5,.47,;-1.04,.95,;.11,-.08,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-4.29,-1.51,;-5.75,-1.99,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;4.69,-8.28,;5.61,-9.51,;7.07,-9.01,;7.05,-7.47,;8.28,-6.54,;9.69,-7.15,;9.88,-8.68,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,)| Show InChI InChI=1S/C27H23Cl2N3O4/c28-21-2-1-3-22(29)24(21)25-20(26(36-31-25)14-4-5-14)13-35-23-10-18-9-16(23)12-32(18)17-6-7-19(27(33)34)15(8-17)11-30/h1-3,6-8,14,16,18,23H,4-5,9-10,12-13H2,(H,33,34)/t16-,18-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465399
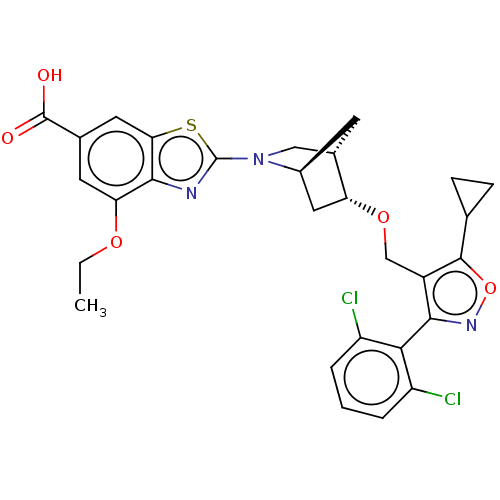
(US10793568, Compound I-18)Show SMILES CCOc1cc(cc2sc(nc12)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)C(O)=O |wU:18.22,14.16,wD:16.17,THB:19:18:13.12:15,9:12:18.17:15,(-4,2.31,;-2.67,1.54,;-1.33,2.31,;0,1.54,;1.33,2.31,;2.67,1.54,;2.67,-0,;1.33,-.77,;1.01,-2.28,;-.52,-2.44,;-1.14,-1.03,;0,-0,;-1.29,-3.77,;-2.53,-4.12,;-2.21,-5.81,;-3.16,-7.04,;-.91,-5.25,;.59,-5.87,;-.79,-6.16,;-.81,-7.7,;.51,-8.49,;.49,-10.03,;-.77,-10.92,;-.32,-12.39,;1.22,-12.41,;1.72,-10.96,;3.19,-10.5,;4.32,-11.55,;3.98,-13.05,;5.79,-11.09,;6.13,-9.59,;5,-8.55,;3.53,-9,;2.4,-7.95,;-2.23,-10.42,;-3.74,-10.72,;-3.24,-9.26,;4,2.31,;5.33,1.54,;4,3.85,)| Show InChI InChI=1S/C29H27Cl2N3O5S/c1-2-37-22-9-15(28(35)36)10-23-26(22)32-29(40-23)34-12-16-8-17(34)11-21(16)38-13-18-25(33-39-27(18)14-6-7-14)24-19(30)4-3-5-20(24)31/h3-5,9-10,14,16-17,21H,2,6-8,11-13H2,1H3,(H,35,36)/t16-,17-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465473
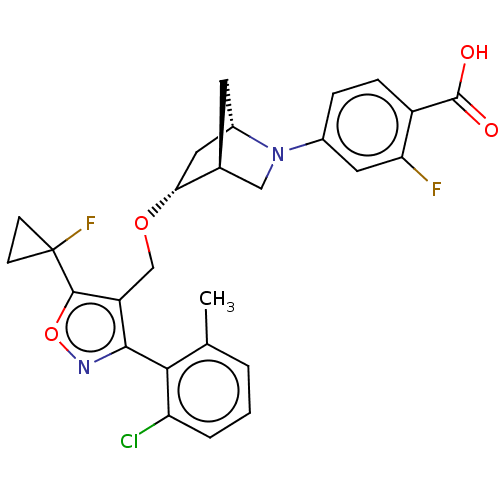
(US10793568, Compound I-124)Show SMILES Cc1cccc(Cl)c1-c1noc(c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc(C(O)=O)c(F)c1)C1(F)CC1 |wU:15.16,19.20,wD:17.19,TLB:22:21:15.16:18,14:15:20.21:18,(-2.51,-3.84,;-4.04,-4.01,;-4.67,-5.42,;-6.2,-5.58,;-7.11,-4.33,;-6.48,-2.93,;-7.39,-1.68,;-4.95,-2.76,;-4.33,-1.36,;-5.1,-.02,;-4.07,1.12,;-2.66,.5,;-2.82,-1.03,;-1.68,-2.06,;-.21,-1.58,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;2.55,-6.43,;3.05,-7.88,;4.56,-8.18,;5.06,-9.64,;4.05,-10.8,;6.57,-9.93,;5.57,-7.02,;7.09,-7.31,;5.07,-5.56,;-1.33,1.27,;-1.86,2.72,;.19,1.54,;-.34,.09,)| Show InChI InChI=1S/C27H25ClF2N2O4/c1-14-3-2-4-20(28)23(14)24-19(25(36-31-24)27(30)7-8-27)13-35-22-11-17-9-15(22)12-32(17)16-5-6-18(26(33)34)21(29)10-16/h2-6,10,15,17,22H,7-9,11-13H2,1H3,(H,33,34)/t15-,17-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465515
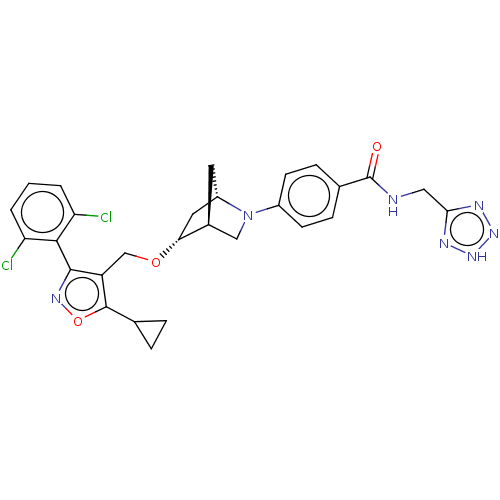
(US10793568, Compound I-164)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc(cc1)C(=O)NCc1nn[nH]n1 |wU:18.20,22.24,wD:20.23,TLB:17:18:23.24:21,25:24:18.19:21,(-7.39,-1.68,;-6.48,-2.93,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.04,-4.01,;-2.51,-3.84,;-4.95,-2.76,;-4.33,-1.35,;-5.1,-.02,;-4.07,1.12,;-2.66,.5,;-1.33,1.27,;-.56,2.61,;.21,1.27,;-2.82,-1.03,;-1.68,-2.06,;-.21,-1.58,;.93,-2.61,;.25,-3.84,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.26,;5.07,-5.56,;5.57,-7.02,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;5.06,-9.63,;6.57,-9.93,;4.05,-10.8,;4.55,-12.25,;6.06,-12.55,;7.19,-11.5,;8.53,-12.25,;8.24,-13.76,;6.71,-13.94,)| Show InChI InChI=1S/C28H27Cl2N7O3/c29-21-2-1-3-22(30)25(21)26-20(27(40-34-26)15-4-5-15)14-39-23-11-19-10-17(23)13-37(19)18-8-6-16(7-9-18)28(38)31-12-24-32-35-36-33-24/h1-3,6-9,15,17,19,23H,4-5,10-14H2,(H,31,38)(H,32,33,35,36)/t17-,19-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250860
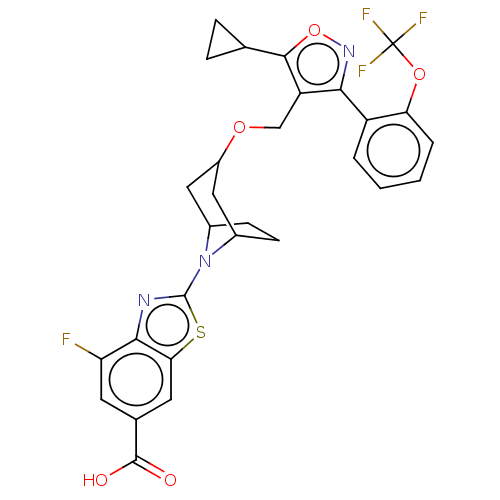
(CHEMBL4062567)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1 |THB:21:19:15.16:13| Show InChI InChI=1S/C29H25F4N3O5S/c30-21-9-15(27(37)38)10-23-25(21)34-28(42-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-41-26(20)14-5-6-14)19-3-1-2-4-22(19)40-29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465464
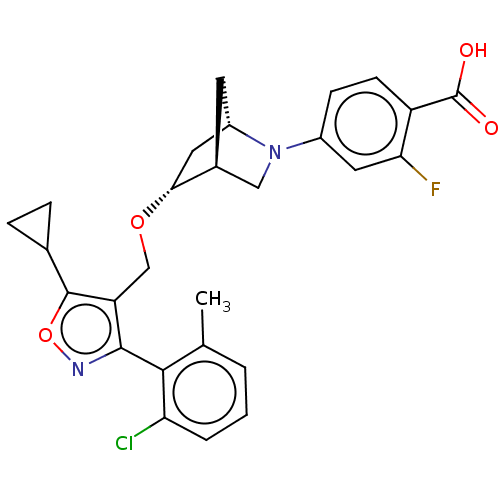
(US10793568, Compound I-115)Show SMILES Cc1cccc(Cl)c1-c1noc(C2CC2)c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc(C(O)=O)c(F)c1 |wU:18.20,22.24,wD:20.23,TLB:25:24:18.19:21,17:18:23.24:21,(-2.51,-3.84,;-4.04,-4.01,;-4.67,-5.42,;-6.2,-5.58,;-7.11,-4.33,;-6.48,-2.93,;-7.39,-1.68,;-4.95,-2.76,;-4.33,-1.36,;-5.1,-.02,;-4.07,1.12,;-2.66,.5,;-1.33,1.27,;-.56,2.61,;.21,1.27,;-2.82,-1.03,;-1.68,-2.06,;-.21,-1.58,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;2.55,-6.43,;3.05,-7.88,;4.56,-8.18,;5.06,-9.64,;4.05,-10.8,;6.57,-9.93,;5.57,-7.02,;7.09,-7.31,;5.07,-5.56,)| Show InChI InChI=1S/C27H26ClFN2O4/c1-14-3-2-4-21(28)24(14)25-20(26(35-30-25)15-5-6-15)13-34-23-11-18-9-16(23)12-31(18)17-7-8-19(27(32)33)22(29)10-17/h2-4,7-8,10,15-16,18,23H,5-6,9,11-13H2,1H3,(H,32,33)/t16-,18-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465576
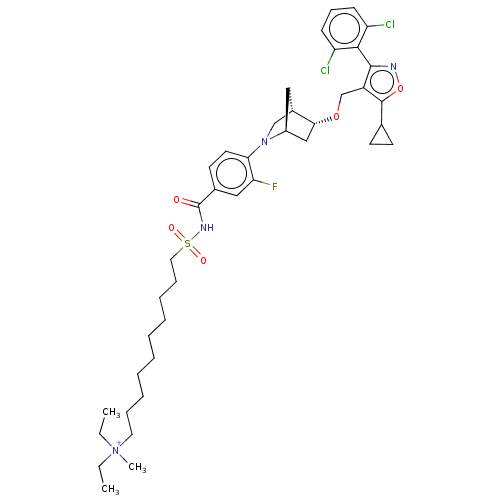
(US10793568, Compound I-226)Show SMILES CC[N+](C)(CC)CCCCCCCCCCS(=O)(=O)NC(=O)c1ccc(N2C[C@@H]3C[C@H]2C[C@H]3OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)c(F)c1 |wU:32.34,28.28,wD:30.29,THB:33:32:27.26:29,25:26:32.31:29,(19.64,-23.36,;18.13,-23.06,;17.63,-21.61,;16.17,-22.1,;19.08,-21.11,;20.24,-22.12,;17.13,-20.15,;15.61,-19.85,;15.12,-18.4,;13.6,-18.1,;13.1,-16.64,;11.59,-16.35,;11.09,-14.89,;9.58,-14.6,;9.08,-13.14,;7.57,-12.84,;7.07,-11.39,;5.62,-11.89,;8.53,-10.89,;6.57,-9.93,;5.06,-9.63,;4.05,-10.79,;4.56,-8.18,;3.05,-7.88,;2.55,-6.42,;3.56,-5.26,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.84,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.35,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.05,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,;5.07,-5.56,;6.09,-4.4,;5.57,-7.02,)| Show InChI InChI=1S/C41H55Cl2FN4O5S/c1-4-48(3,5-2)21-12-10-8-6-7-9-11-13-22-54(50,51)46-41(49)29-19-20-36(35(44)24-29)47-26-30-23-31(47)25-37(30)52-27-32-39(45-53-40(32)28-17-18-28)38-33(42)15-14-16-34(38)43/h14-16,19-20,24,28,30-31,37H,4-13,17-18,21-23,25-27H2,1-3H3/p+1/t30-,31-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465471
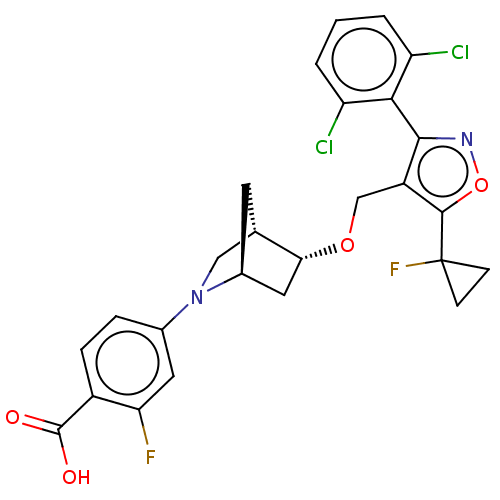
(US10793568, Compound I-122)Show SMILES OC(=O)c1ccc(cc1F)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(noc1C1(F)CC1)-c1c(Cl)cccc1Cl |wU:16.19,12.13,wD:14.14,THB:6:10:16.15:13,17:16:11.10:13,(4.05,-10.8,;5.06,-9.64,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;7.09,-7.31,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-4.33,-1.36,;-5.1,-.02,;-4.07,1.12,;-2.66,.5,;-1.33,1.27,;-1.86,2.72,;.19,1.54,;-.34,.09,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,)| Show InChI InChI=1S/C26H22Cl2F2N2O4/c27-18-2-1-3-19(28)22(18)23-17(24(36-31-23)26(30)6-7-26)12-35-21-10-15-8-13(21)11-32(15)14-4-5-16(25(33)34)20(29)9-14/h1-5,9,13,15,21H,6-8,10-12H2,(H,33,34)/t13-,15-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250860

(CHEMBL4062567)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1 |THB:21:19:15.16:13| Show InChI InChI=1S/C29H25F4N3O5S/c30-21-9-15(27(37)38)10-23-25(21)34-28(42-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-41-26(20)14-5-6-14)19-3-1-2-4-22(19)40-29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250868
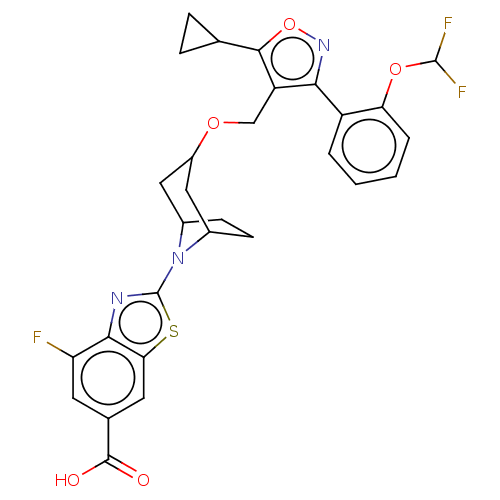
(CHEMBL4083548)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)F)C1CC1 |THB:9:13:15.16:18.19.20,21:19:13:15.16| Show InChI InChI=1S/C29H26F3N3O5S/c30-21-9-15(27(36)37)10-23-25(21)33-29(41-23)35-16-7-8-17(35)12-18(11-16)38-13-20-24(34-40-26(20)14-5-6-14)19-3-1-2-4-22(19)39-28(31)32/h1-4,9-10,14,16-18,28H,5-8,11-13H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50258109
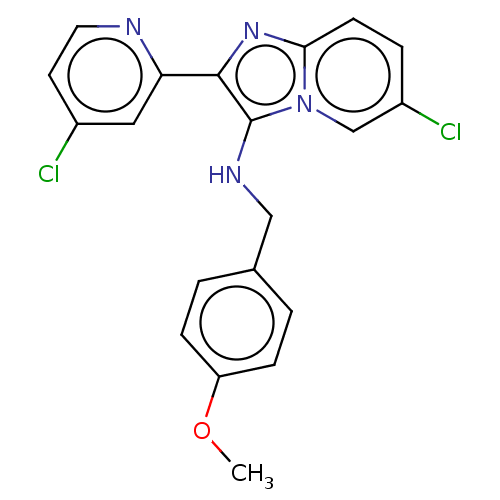
(CHEMBL4077016)Show SMILES COc1ccc(CNc2c(nc3ccc(Cl)cn23)-c2cc(Cl)ccn2)cc1 Show InChI InChI=1S/C20H16Cl2N4O/c1-27-16-5-2-13(3-6-16)11-24-20-19(17-10-14(21)8-9-23-17)25-18-7-4-15(22)12-26(18)20/h2-10,12,24H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry, Goethe-University Frankfurt , Max-von-Laue-Strasse 9, D-60438 Frankfurt am Main, Germany.
Curated by ChEMBL
| Assay Description
Transactivation of human FXR expressed in human HeLa cells co-expressing BSEP after 24 hrs by dual-glo luciferase reporter gene assay |
J Med Chem 60: 7199-7205 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00903
BindingDB Entry DOI: 10.7270/Q2736TBH |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465575

(US10793568, Compound I-225)Show SMILES COCC(=O)NCCCCCCCCCCS(=O)(=O)NC(=O)c1ccc(N2C[C@@H]3C[C@H]2C[C@H]3OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)c(F)c1 |wU:32.34,28.28,wD:30.29,THB:33:32:27.26:29,25:26:32.31:29,(1.46,-32.03,;.96,-30.57,;1.98,-29.41,;1.48,-27.96,;-.04,-27.66,;2.49,-26.8,;1.99,-25.34,;3,-24.18,;2.5,-22.72,;3.51,-21.56,;3.01,-20.1,;4.02,-18.94,;3.53,-17.49,;4.54,-16.33,;4.04,-14.87,;5.05,-13.71,;4.55,-12.25,;3.09,-12.75,;6.01,-11.75,;4.05,-10.79,;5.06,-9.63,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.55,-6.42,;3.56,-5.26,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.84,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.35,;-4.95,-2.76,;-6.48,-2.92,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,;5.07,-5.56,;6.09,-4.4,;5.57,-7.02,)| Show InChI InChI=1S/C39H49Cl2FN4O7S/c1-51-24-35(47)43-17-8-6-4-2-3-5-7-9-18-54(49,50)45-39(48)26-15-16-33(32(42)20-26)46-22-27-19-28(46)21-34(27)52-23-29-37(44-53-38(29)25-13-14-25)36-30(40)11-10-12-31(36)41/h10-12,15-16,20,25,27-28,34H,2-9,13-14,17-19,21-24H2,1H3,(H,43,47)(H,45,48)/t27-,28-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465457
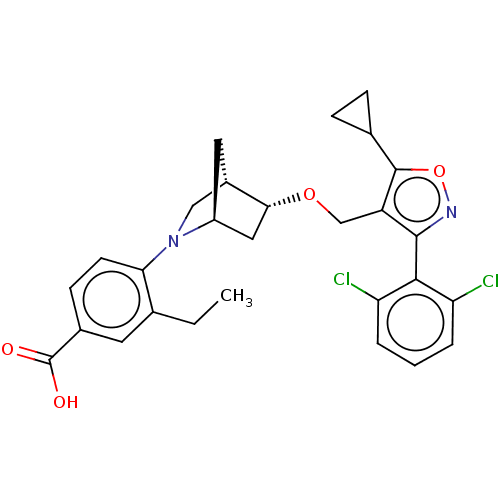
(US10793568, Compound I-109)Show SMILES CCc1cc(ccc1N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)C(O)=O |wU:14.17,10.11,wD:12.12,THB:15:14:9.8:11,7:8:14.13:11,(7.6,-4.7,;6.09,-4.4,;5.07,-5.56,;5.57,-7.02,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.26,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,;5.06,-9.63,;4.05,-10.8,;6.57,-9.93,)| Show InChI InChI=1S/C28H28Cl2N2O4/c1-2-15-10-17(28(33)34)8-9-23(15)32-13-18-11-19(32)12-24(18)35-14-20-26(31-36-27(20)16-6-7-16)25-21(29)4-3-5-22(25)30/h3-5,8-10,16,18-19,24H,2,6-7,11-14H2,1H3,(H,33,34)/t18-,19-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465521
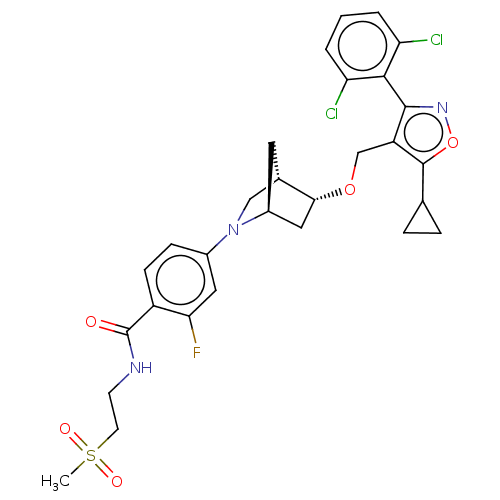
(US10793568, Compound I-170)Show SMILES CS(=O)(=O)CCNC(=O)c1ccc(cc1F)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:22.25,18.19,wD:20.20,THB:23:22:17.16:19,12:16:22.21:19,(-.98,-8.45,;.53,-8.75,;.82,-7.24,;.23,-10.26,;2.04,-9.04,;2.54,-10.5,;4.05,-10.8,;5.06,-9.64,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;7.09,-7.31,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;.11,-.08,;-1.04,.95,;-2.55,.63,;-3.32,1.96,;-2.29,3.11,;-.88,2.48,;.45,3.26,;1.79,2.49,;1.79,.95,;3.12,3.26,;3.12,4.8,;1.78,5.57,;.45,4.8,;-.89,5.56,;-3.17,-.78,;-3.01,-2.31,;-4.41,-1.69,)| Show InChI InChI=1S/C29H30Cl2FN3O5S/c1-41(37,38)10-9-33-29(36)20-8-7-18(12-24(20)32)35-14-17-11-19(35)13-25(17)39-15-21-27(34-40-28(21)16-5-6-16)26-22(30)3-2-4-23(26)31/h2-4,7-8,12,16-17,19,25H,5-6,9-11,13-15H2,1H3,(H,33,36)/t17-,19-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465453
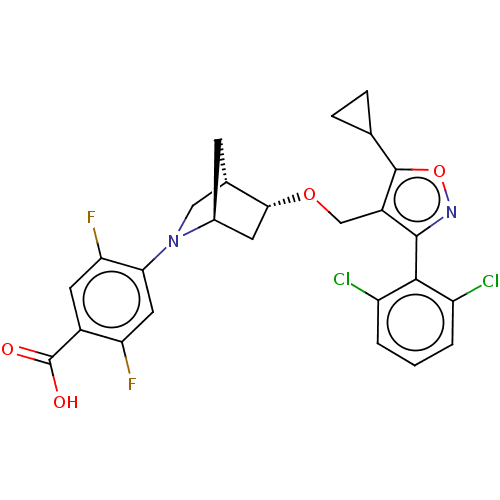
(US10793568, Compound I-105)Show SMILES OC(=O)c1cc(F)c(cc1F)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:17.20,13.14,wD:15.15,THB:18:17:12.11:14,7:11:17.16:14,(4.05,-10.8,;5.06,-9.63,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;1.04,-6.13,;3.56,-5.26,;5.07,-5.56,;5.57,-7.02,;7.09,-7.31,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C26H22Cl2F2N2O4/c27-17-2-1-3-18(28)23(17)24-16(25(36-31-24)12-4-5-12)11-35-22-7-14-6-13(22)10-32(14)21-9-19(29)15(26(33)34)8-20(21)30/h1-3,8-9,12-14,22H,4-7,10-11H2,(H,33,34)/t13-,14-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465452
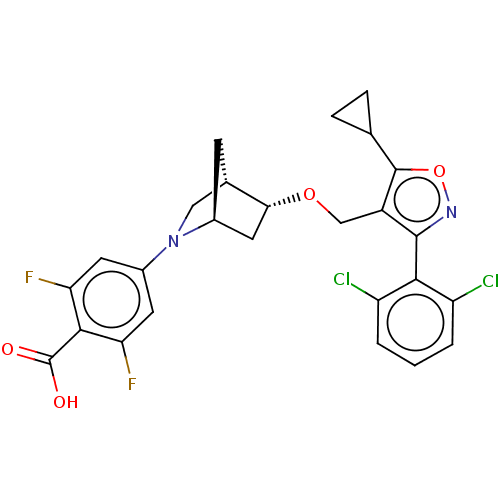
(US10793568, Compound I-104)Show SMILES OC(=O)c1c(F)cc(cc1F)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:17.20,13.14,wD:15.15,THB:18:17:12.11:14,7:11:17.16:14,(4.05,-10.8,;5.06,-9.63,;6.57,-9.93,;4.56,-8.18,;3.05,-7.88,;2.04,-9.04,;2.55,-6.43,;3.56,-5.26,;5.07,-5.56,;5.57,-7.02,;7.09,-7.31,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C26H22Cl2F2N2O4/c27-17-2-1-3-18(28)22(17)24-16(25(36-31-24)12-4-5-12)11-35-21-9-14-6-13(21)10-32(14)15-7-19(29)23(26(33)34)20(30)8-15/h1-3,7-8,12-14,21H,4-6,9-11H2,(H,33,34)/t13-,14-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465460
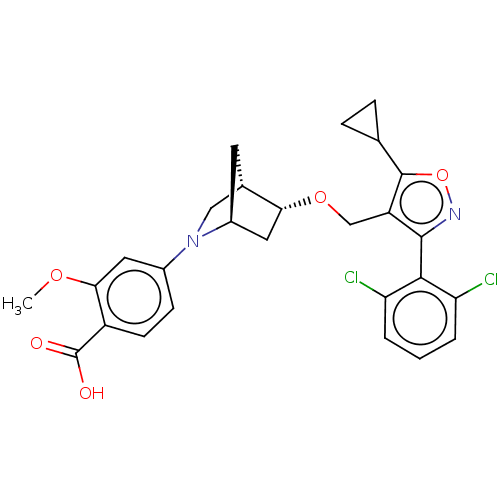
(US10793568, Compound I-112)Show SMILES COc1cc(ccc1C(O)=O)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:17.20,13.14,wD:15.15,THB:18:17:12.11:14,4:11:17.16:14,(7.58,-8.77,;7.09,-7.31,;5.57,-7.02,;5.07,-5.56,;3.56,-5.26,;2.55,-6.43,;3.05,-7.88,;4.56,-8.18,;5.06,-9.63,;4.05,-10.8,;6.57,-9.93,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C27H26Cl2N2O5/c1-34-23-10-16(7-8-18(23)27(32)33)31-12-15-9-17(31)11-22(15)35-13-19-25(30-36-26(19)14-5-6-14)24-20(28)3-2-4-21(24)29/h2-4,7-8,10,14-15,17,22H,5-6,9,11-13H2,1H3,(H,32,33)/t15-,17-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465566
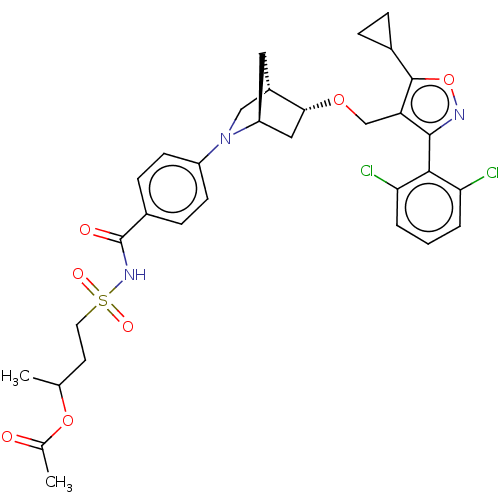
(US10793568, Compound I-216)Show SMILES CC(CCS(=O)(=O)NC(=O)c1ccc(cc1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)OC(C)=O |wU:20.20,22.25,wD:18.19,TLB:13:16:21.22:19,23:22:16.17:19,(-9.7,5.14,;-10.02,3.63,;-8.87,2.6,;-7.41,3.08,;-6.26,2.05,;-7.29,.91,;-5.23,3.2,;-5.11,1.02,;-3.65,1.5,;-3.33,3.01,;-2.5,.47,;-1.04,.95,;.11,-.08,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;4.69,-8.28,;5.61,-9.51,;7.07,-9.01,;7.05,-7.47,;8.28,-6.54,;9.69,-7.15,;9.88,-8.68,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,;-11.48,3.15,;-12.63,4.18,;-14.09,3.71,;-12.31,5.69,)| Show InChI InChI=1S/C32H35Cl2N3O7S/c1-18(43-19(2)38)12-13-45(40,41)36-32(39)21-8-10-23(11-9-21)37-16-22-14-24(37)15-28(22)42-17-25-30(35-44-31(25)20-6-7-20)29-26(33)4-3-5-27(29)34/h3-5,8-11,18,20,22,24,28H,6-7,12-17H2,1-2H3,(H,36,39)/t18?,22-,24-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465487
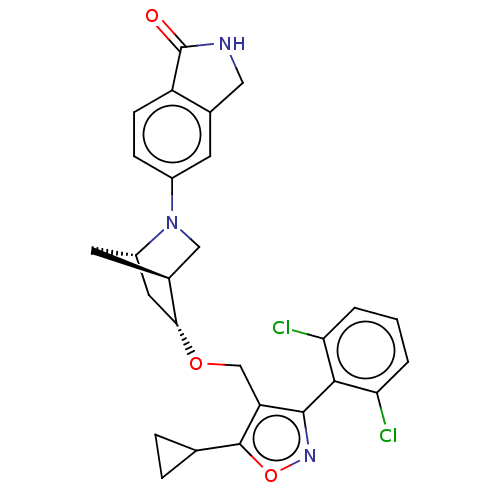
(US10793568, Compound I-135)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc2C(=O)NCc2c1 |wU:18.20,22.24,wD:20.23,TLB:17:18:23.24:21,25:24:18.19:21,(10.53,-7.71,;10.08,-6.24,;11.13,-5.11,;10.67,-3.64,;9.17,-3.3,;8.12,-4.43,;6.62,-4.08,;8.58,-5.9,;7.53,-7.03,;7.83,-8.54,;6.48,-9.29,;5.35,-8.24,;3.84,-8.54,;2.68,-9.55,;2.38,-8.04,;6,-6.84,;5.25,-5.5,;3.71,-5.48,;2.96,-4.13,;4,-3.19,;2.4,-3.41,;1.35,-6.08,;1.56,-4.54,;.43,-3.24,;1.33,-2.31,;1.33,-.77,;2.67,,;2.67,1.54,;1.33,2.31,;1.01,3.82,;2.04,4.96,;-.52,3.98,;-1.14,2.57,;,1.54,;)| Show InChI InChI=1S/C27H25Cl2N3O3/c28-21-2-1-3-22(29)24(21)25-20(26(35-31-25)14-4-5-14)13-34-23-10-18-9-16(23)12-32(18)17-6-7-19-15(8-17)11-30-27(19)33/h1-3,6-8,14,16,18,23H,4-5,9-13H2,(H,30,33)/t16-,18-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465563
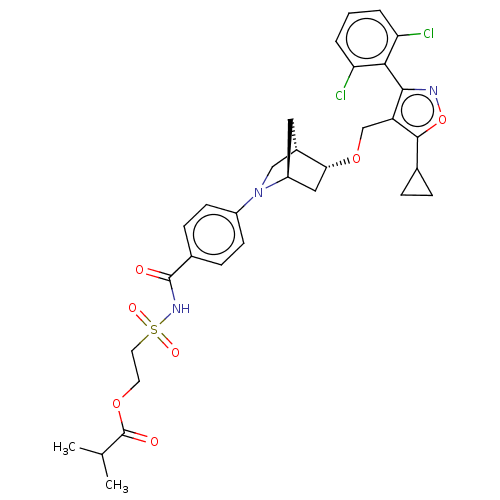
(US10793568, Compound I-213)Show SMILES CC(C)C(=O)OCCS(=O)(=O)NC(=O)c1ccc(cc1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:24.24,26.29,wD:22.23,TLB:17:20:25.26:23,27:26:20.21:23,(-14.09,3.71,;-12.63,4.18,;-12.31,5.69,;-11.48,3.15,;-11.8,1.65,;-10.02,3.63,;-8.87,2.6,;-7.41,3.08,;-6.26,2.05,;-7.29,.91,;-5.23,3.2,;-5.11,1.02,;-3.65,1.5,;-3.33,3.01,;-2.5,.47,;-1.04,.95,;.11,-.08,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;4.69,-8.28,;5.61,-9.51,;7.07,-9.01,;7.05,-7.47,;8.28,-6.54,;9.69,-7.15,;9.88,-8.68,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,)| Show InChI InChI=1S/C32H35Cl2N3O7S/c1-18(2)32(39)42-12-13-45(40,41)36-31(38)20-8-10-22(11-9-20)37-16-21-14-23(37)15-27(21)43-17-24-29(35-44-30(24)19-6-7-19)28-25(33)4-3-5-26(28)34/h3-5,8-11,18-19,21,23,27H,6-7,12-17H2,1-2H3,(H,36,38)/t21-,23-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50241176
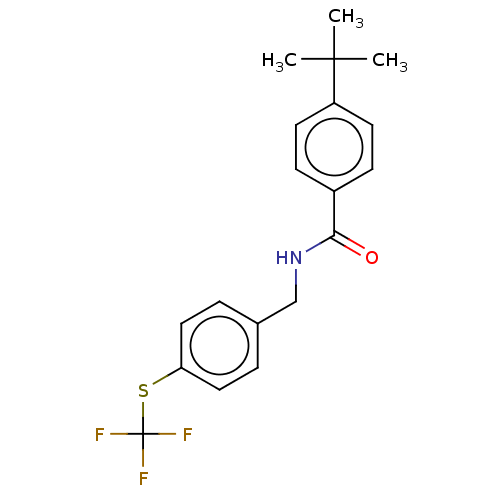
(CHEMBL4074393)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)NCc1ccc(SC(F)(F)F)cc1 Show InChI InChI=1S/C19H20F3NOS/c1-18(2,3)15-8-6-14(7-9-15)17(24)23-12-13-4-10-16(11-5-13)25-19(20,21)22/h4-11H,12H2,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 60: 7703-7724 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00398
BindingDB Entry DOI: 10.7270/Q2N3003W |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50553984
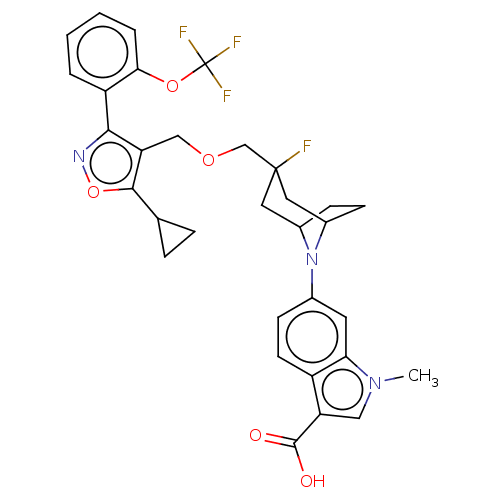
(CHEMBL4751757)Show SMILES Cn1cc(C(O)=O)c2ccc(cc12)N1C2CCC1CC(F)(COCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C2 |TLB:10:13:19.43.18:15.16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human FXR expressed in HEK293 cells measured after 18 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01065
BindingDB Entry DOI: 10.7270/Q2PN9983 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250870
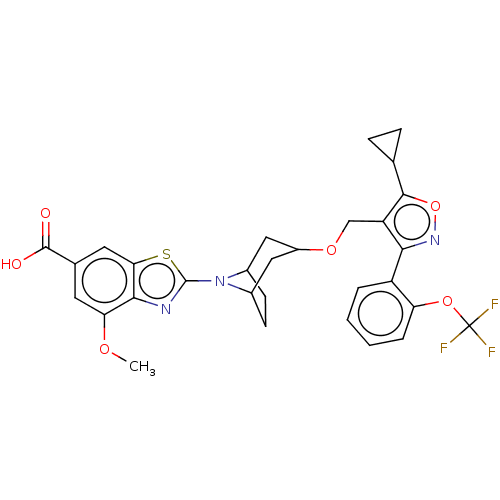
(CHEMBL4076774)Show SMILES COc1cc(cc2sc(nc12)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C(O)=O |THB:8:11:13.14:16.17.18,19:17:11:13.14| Show InChI InChI=1S/C30H28F3N3O6S/c1-39-23-10-16(28(37)38)11-24-26(23)34-29(43-24)36-17-8-9-18(36)13-19(12-17)40-14-21-25(35-42-27(21)15-6-7-15)20-4-2-3-5-22(20)41-30(31,32)33/h2-5,10-11,15,17-19H,6-9,12-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250869
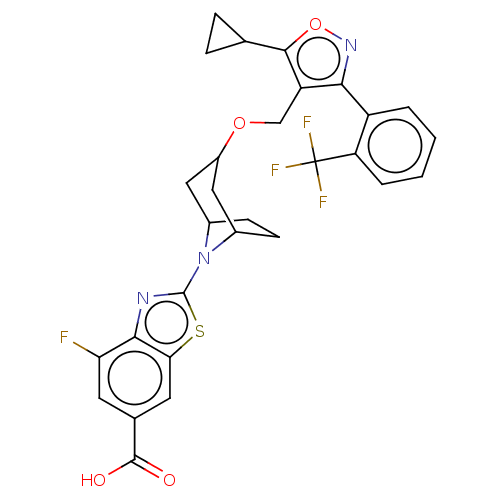
(CHEMBL4061776)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1C(F)(F)F)C1CC1 |THB:9:13:15.16:18.19.20,21:19:13:15.16| Show InChI InChI=1S/C29H25F4N3O4S/c30-22-9-15(27(37)38)10-23-25(22)34-28(41-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-40-26(20)14-5-6-14)19-3-1-2-4-21(19)29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250867
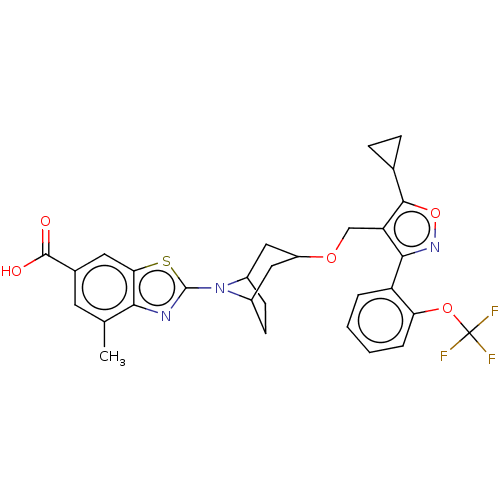
(CHEMBL4103787)Show SMILES Cc1cc(cc2sc(nc12)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C(O)=O |THB:7:10:12.13:15.16.17,18:16:10:12.13| Show InChI InChI=1S/C30H28F3N3O5S/c1-15-10-17(28(37)38)11-24-25(15)34-29(42-24)36-18-8-9-19(36)13-20(12-18)39-14-22-26(35-41-27(22)16-6-7-16)21-4-2-3-5-23(21)40-30(31,32)33/h2-5,10-11,16,18-20H,6-9,12-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50527040
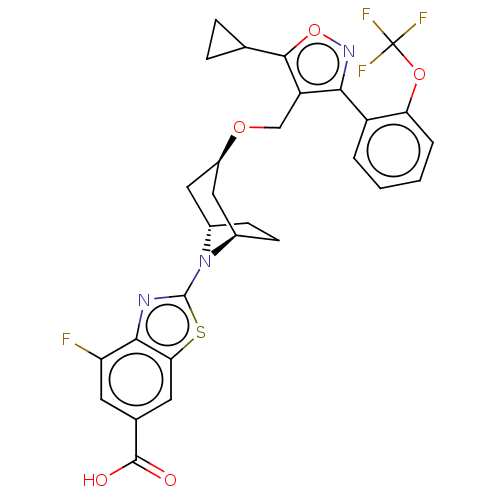
(LJN-452 | LJN452 | NVP-LJN452-NXA | Tropifexor)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)N2c1nc2c(F)cc(cc2s1)C(O)=O |r,TLB:31:30:2.3:6.7.8| Show InChI InChI=1S/C29H25F4N3O5S/c30-21-9-15(27(37)38)10-23-25(21)34-28(42-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-41-26(20)14-5-6-14)19-3-1-2-4-22(19)40-29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38)/t16-,17+,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Agonist activity at GST-tagged human FXR-LBD (193 to 472 residues) using biotinylated SRC-1 peptide as substrate incubated for 1 hrs by HTRF assay |
J Med Chem 63: 3868-3880 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01621
BindingDB Entry DOI: 10.7270/Q2K077QV |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50553985
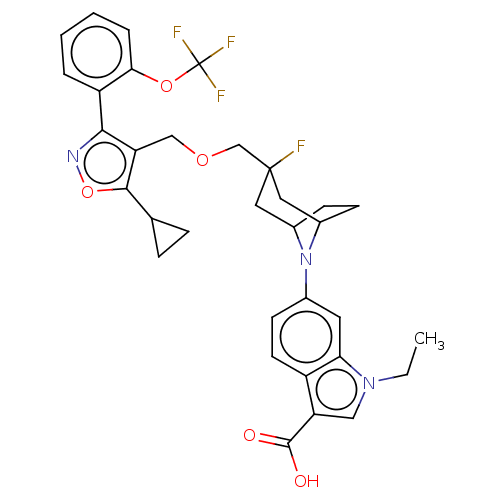
(CHEMBL4781153)Show SMILES CCn1cc(C(O)=O)c2ccc(cc12)N1C2CCC1CC(F)(COCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C2 |TLB:11:14:20.44.19:16.17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human FXR expressed in HEK293 cells measured after 18 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01065
BindingDB Entry DOI: 10.7270/Q2PN9983 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465459
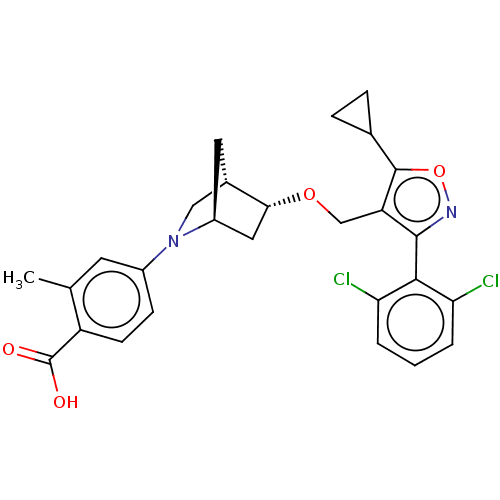
(US10793568, Compound I-111)Show SMILES Cc1cc(ccc1C(O)=O)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:16.19,12.13,wD:14.14,THB:17:16:11.10:13,3:10:16.15:13,(7.09,-7.31,;5.57,-7.02,;5.07,-5.56,;3.56,-5.26,;2.55,-6.43,;3.05,-7.88,;4.56,-8.18,;5.06,-9.63,;4.05,-10.8,;6.57,-9.93,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.42,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C27H26Cl2N2O4/c1-14-9-17(7-8-19(14)27(32)33)31-12-16-10-18(31)11-23(16)34-13-20-25(30-35-26(20)15-5-6-15)24-21(28)3-2-4-22(24)29/h2-4,7-9,15-16,18,23H,5-6,10-13H2,1H3,(H,32,33)/t16-,18-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465477
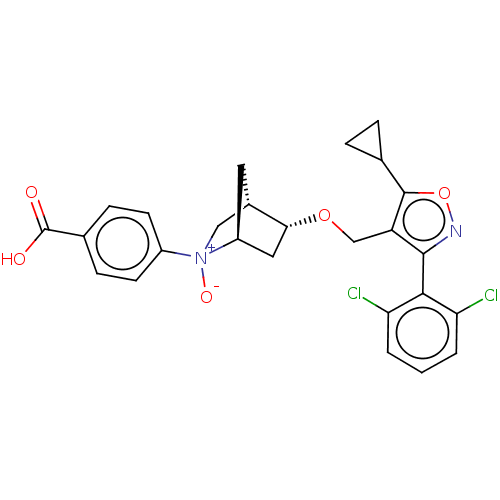
(US10793568, Compound I-127)Show SMILES OC(=O)c1ccc(cc1)[N+]1([O-])C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:14.14,16.19,wD:12.13,TLB:17:16:9.11:13,10:9:15.16:13,6:9:15.16:13,(-5.76,-.38,;-5.17,-1.8,;-6.11,-3.02,;-3.65,-2,;-2.71,-.78,;-1.18,-.99,;-.59,-2.41,;-1.53,-3.63,;-3.06,-3.43,;.93,-2.61,;.57,-1.12,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;4.69,-8.28,;5.61,-9.51,;7.07,-9.01,;7.05,-7.47,;8.28,-6.54,;9.69,-7.15,;9.88,-8.68,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,)| Show InChI InChI=1S/C26H24Cl2N2O5/c27-20-2-1-3-21(28)23(20)24-19(25(35-29-24)14-4-5-14)13-34-22-11-18-10-16(22)12-30(18,33)17-8-6-15(7-9-17)26(31)32/h1-3,6-9,14,16,18,22H,4-5,10-13H2,(H,31,32)/t16-,18-,22+,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50544020
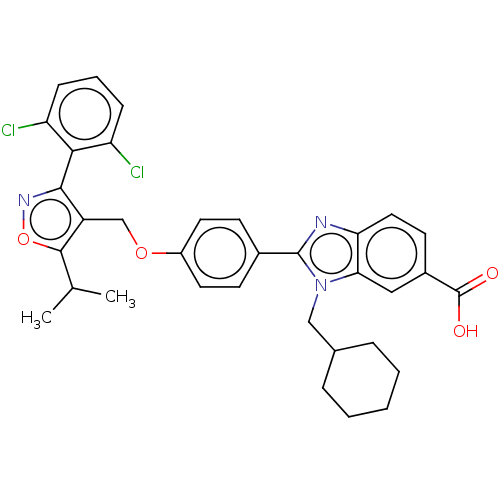
(CHEMBL4638213)Show SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1nc2ccc(cc2n1CC1CCCCC1)C(O)=O)-c1c(Cl)cccc1Cl |(36.95,-14.4,;37.58,-12.99,;39.11,-12.83,;36.68,-11.75,;35.13,-11.75,;34.66,-10.28,;35.9,-9.37,;37.15,-10.28,;38.62,-9.8,;39.76,-10.84,;41.23,-10.36,;42.38,-11.39,;43.83,-10.92,;44.16,-9.41,;43.01,-8.37,;41.54,-8.86,;45.63,-8.93,;46.87,-9.83,;48.11,-8.92,;49.61,-9.24,;50.64,-8.09,;50.16,-6.63,;48.66,-6.32,;47.64,-7.46,;46.1,-7.46,;45.19,-6.22,;45.81,-4.81,;47.34,-4.66,;47.96,-3.26,;47.07,-2.02,;45.54,-2.18,;44.91,-3.58,;51.18,-5.47,;52.69,-5.79,;50.7,-4.01,;35.89,-7.83,;37.23,-7.05,;38.57,-7.83,;37.23,-5.5,;35.89,-4.74,;34.56,-5.51,;34.56,-7.06,;33.23,-7.83,)| Show InChI InChI=1S/C34H33Cl2N3O4/c1-20(2)32-25(31(38-43-32)30-26(35)9-6-10-27(30)36)19-42-24-14-11-22(12-15-24)33-37-28-16-13-23(34(40)41)17-29(28)39(33)18-21-7-4-3-5-8-21/h6,9-17,20-21H,3-5,7-8,18-19H2,1-2H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in human HuH7 cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115512
BindingDB Entry DOI: 10.7270/Q20G3PQ8 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250869

(CHEMBL4061776)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1C(F)(F)F)C1CC1 |THB:9:13:15.16:18.19.20,21:19:13:15.16| Show InChI InChI=1S/C29H25F4N3O4S/c30-22-9-15(27(37)38)10-23-25(22)34-28(41-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-40-26(20)14-5-6-14)19-3-1-2-4-21(19)29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250866
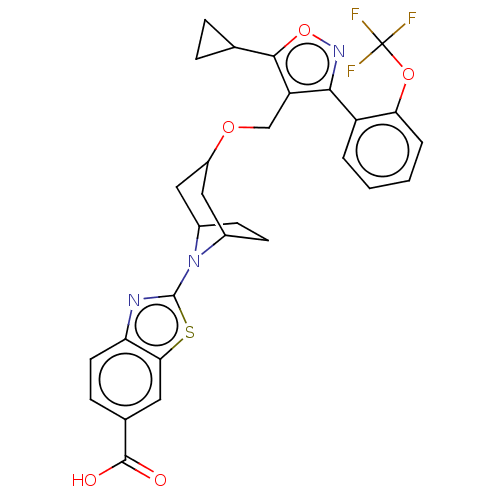
(CHEMBL4084716)Show SMILES OC(=O)c1ccc2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1 |THB:8:12:14.15:17.18.19,20:18:12:14.15| Show InChI InChI=1S/C29H26F3N3O5S/c30-29(31,32)39-23-4-2-1-3-20(23)25-21(26(40-34-25)15-5-6-15)14-38-19-12-17-8-9-18(13-19)35(17)28-33-22-10-7-16(27(36)37)11-24(22)41-28/h1-4,7,10-11,15,17-19H,5-6,8-9,12-14H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250870

(CHEMBL4076774)Show SMILES COc1cc(cc2sc(nc12)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C(O)=O |THB:8:11:13.14:16.17.18,19:17:11:13.14| Show InChI InChI=1S/C30H28F3N3O6S/c1-39-23-10-16(28(37)38)11-24-26(23)34-29(43-24)36-17-8-9-18(36)13-19(12-17)40-14-21-25(35-42-27(21)15-6-7-15)20-4-2-3-5-22(20)41-30(31,32)33/h2-5,10-11,15,17-19H,6-9,12-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465562
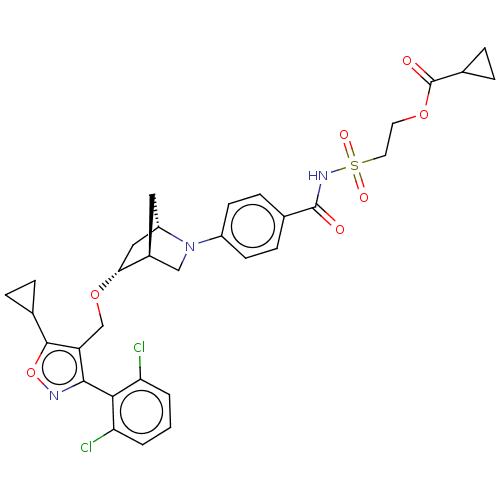
(US10793568, Compound I-212)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc(cc1)C(=O)NS(=O)(=O)CCOC(=O)C1CC1 |wU:20.23,18.20,wD:22.24,THB:25:24:19.18:21,17:18:24.23:21,(9.88,-8.68,;9.69,-7.15,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;8.28,-6.54,;7.05,-7.47,;7.07,-9.01,;5.61,-9.51,;4.69,-8.28,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,;5.57,-7.02,;5.07,-5.56,;3.56,-5.27,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;.11,-.08,;-1.04,.95,;-2.5,.47,;-2.82,-1.03,;-1.68,-2.06,;-3.65,1.5,;-3.33,3.01,;-5.11,1.02,;-6.26,2.05,;-7.29,.91,;-5.23,3.2,;-7.41,3.08,;-8.87,2.6,;-10.02,3.63,;-11.48,3.15,;-11.8,1.65,;-12.63,4.18,;-14.13,4.5,;-13.1,5.65,)| Show InChI InChI=1S/C32H33Cl2N3O7S/c33-25-2-1-3-26(34)28(25)29-24(30(44-35-29)18-4-5-18)17-43-27-15-23-14-21(27)16-37(23)22-10-8-19(9-11-22)31(38)36-45(40,41)13-12-42-32(39)20-6-7-20/h1-3,8-11,18,20-21,23,27H,4-7,12-17H2,(H,36,38)/t21-,23-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465538
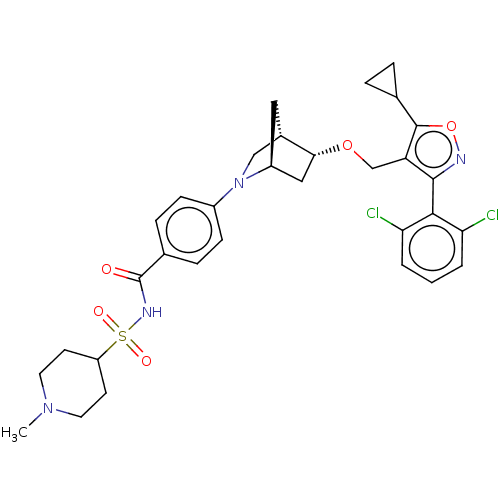
(US10793568, Compound I-187)Show SMILES CN1CCC(CC1)S(=O)(=O)NC(=O)c1ccc(cc1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:25.29,21.23,wD:23.24,THB:26:25:20.19:22,16:19:25.24:22,(9.07,-17.21,;8.57,-15.76,;9.58,-14.6,;9.08,-13.14,;7.57,-12.84,;6.56,-14,;7.06,-15.46,;7.07,-11.39,;5.62,-11.89,;8.53,-10.89,;6.57,-9.93,;5.06,-9.63,;4.05,-10.79,;4.56,-8.18,;5.57,-7.02,;5.07,-5.56,;3.56,-5.26,;2.55,-6.42,;3.05,-7.88,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.84,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.35,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,)| Show InChI InChI=1S/C32H36Cl2N4O5S/c1-37-13-11-24(12-14-37)44(40,41)36-32(39)20-7-9-22(10-8-20)38-17-21-15-23(38)16-28(21)42-18-25-30(35-43-31(25)19-5-6-19)29-26(33)3-2-4-27(29)34/h2-4,7-10,19,21,23-24,28H,5-6,11-18H2,1H3,(H,36,39)/t21-,23-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465524
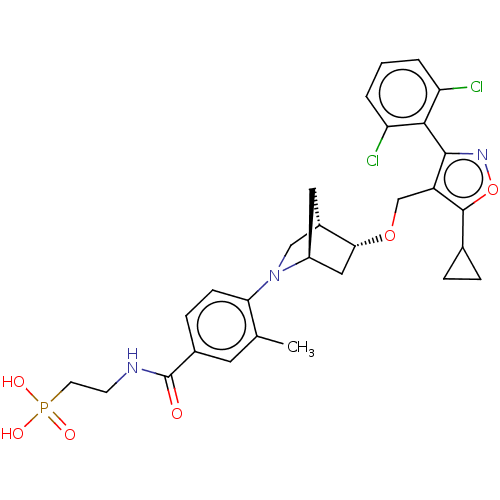
(US10793568, Compound I-173)Show SMILES Cc1cc(ccc1N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1)C(=O)NCCP(O)(O)=O |wU:13.16,9.10,wD:11.11,THB:14:13:8.7:10,6:7:13.12:10,(6.09,-4.4,;5.07,-5.56,;5.57,-7.02,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.27,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;.11,-.08,;-1.04,.95,;-2.55,.63,;-3.32,1.96,;-2.29,3.11,;-.88,2.48,;.45,3.26,;1.79,2.49,;1.79,.95,;3.12,3.26,;3.12,4.8,;1.78,5.57,;.45,4.8,;-.89,5.56,;-3.17,-.78,;-3.01,-2.31,;-4.41,-1.69,;5.06,-9.64,;6.57,-9.93,;4.05,-10.8,;2.54,-10.5,;2.04,-9.04,;.53,-8.75,;.82,-7.24,;.23,-10.26,;-.98,-8.45,)| Show InChI InChI=1S/C29H32Cl2N3O6P/c1-16-11-18(29(35)32-9-10-41(36,37)38)7-8-24(16)34-14-19-12-20(34)13-25(19)39-15-21-27(33-40-28(21)17-5-6-17)26-22(30)3-2-4-23(26)31/h2-4,7-8,11,17,19-20,25H,5-6,9-10,12-15H2,1H3,(H,32,35)(H2,36,37,38)/t19-,20-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465504
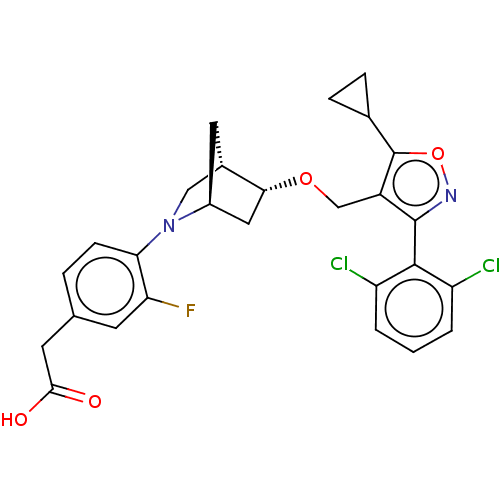
(US10793568, Compound I-153)Show SMILES OC(=O)Cc1ccc(N2C[C@@H]3C[C@H]2C[C@H]3OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)c(F)c1 |wU:14.16,10.10,wD:12.11,THB:15:14:9.8:11,7:8:14.13:11,(2.54,-10.5,;4.05,-10.8,;4.55,-12.25,;5.06,-9.63,;4.56,-8.18,;3.05,-7.88,;2.55,-6.43,;3.56,-5.26,;3.06,-3.81,;3.61,-2.64,;2.13,-1.77,;1.83,-.25,;1.7,-3.12,;.25,-3.85,;.93,-2.61,;-.21,-1.58,;-1.68,-2.06,;-2.82,-1.03,;-2.66,.5,;-4.07,1.12,;-5.1,-.02,;-4.33,-1.36,;-4.95,-2.76,;-6.48,-2.93,;-7.39,-1.68,;-7.11,-4.33,;-6.2,-5.58,;-4.67,-5.41,;-4.04,-4.01,;-2.51,-3.84,;-1.33,1.27,;-.56,2.61,;.21,1.27,;5.07,-5.56,;6.09,-4.4,;5.57,-7.02,)| Show InChI InChI=1S/C27H25Cl2FN2O4/c28-19-2-1-3-20(29)25(19)26-18(27(36-31-26)15-5-6-15)13-35-23-11-17-10-16(23)12-32(17)22-7-4-14(8-21(22)30)9-24(33)34/h1-4,7-8,15-17,23H,5-6,9-13H2,(H,33,34)/t16-,17-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465636
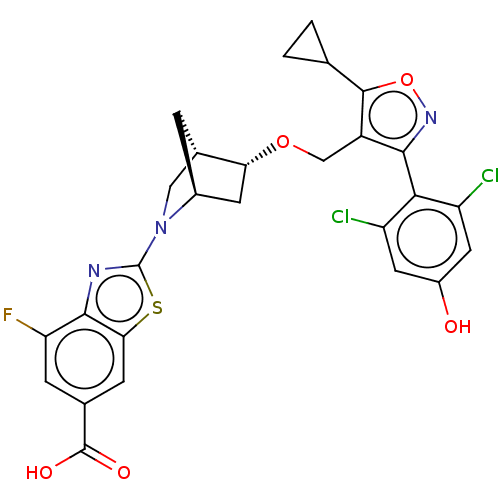
(US10793568, Compound I-287)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cc(O)cc1Cl)C1CC1 |wU:19.23,15.17,wD:17.18,THB:20:19:14.13:16,9:13:19.18:16,(5.33,1.54,;4,2.31,;4,3.85,;2.67,1.54,;1.33,2.31,;0,1.54,;-1.33,2.31,;0,-0,;-1.14,-1.03,;-.52,-2.44,;1.01,-2.28,;1.33,-.77,;2.67,-0,;-1.29,-3.77,;-2.53,-4.12,;-2.21,-5.81,;-3.16,-7.04,;-.91,-5.25,;.59,-5.87,;-.79,-6.16,;-.81,-7.7,;.51,-8.49,;.49,-10.03,;-.77,-10.92,;-.32,-12.39,;1.22,-12.41,;1.72,-10.96,;3.19,-10.5,;4.32,-11.55,;3.98,-13.05,;5.79,-11.09,;6.13,-9.59,;7.6,-9.14,;5,-8.55,;3.53,-9,;2.4,-7.95,;-2.23,-10.42,;-3.74,-10.72,;-3.24,-9.26,)| Show InChI InChI=1S/C27H22Cl2FN3O5S/c28-17-7-15(34)8-18(29)22(17)23-16(25(38-32-23)11-1-2-11)10-37-20-6-14-3-13(20)9-33(14)27-31-24-19(30)4-12(26(35)36)5-21(24)39-27/h4-5,7-8,11,13-14,20,34H,1-3,6,9-10H2,(H,35,36)/t13-,14-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250866

(CHEMBL4084716)Show SMILES OC(=O)c1ccc2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1 |THB:8:12:14.15:17.18.19,20:18:12:14.15| Show InChI InChI=1S/C29H26F3N3O5S/c30-29(31,32)39-23-4-2-1-3-20(23)25-21(26(40-34-25)15-5-6-15)14-38-19-12-17-8-9-18(13-19)35(17)28-33-22-10-7-16(27(36)37)11-24(22)41-28/h1-4,7,10-11,15,17-19H,5-6,8-9,12-14H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in HEK293T cells assessed as BSEP promoter driven cellular transcriptional activity after 24 hrs by luciferas... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50527040

(LJN-452 | LJN452 | NVP-LJN452-NXA | Tropifexor)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)N2c1nc2c(F)cc(cc2s1)C(O)=O |r,TLB:31:30:2.3:6.7.8| Show InChI InChI=1S/C29H25F4N3O5S/c30-21-9-15(27(37)38)10-23-25(21)34-28(42-23)36-16-7-8-17(36)12-18(11-16)39-13-20-24(35-41-26(20)14-5-6-14)19-3-1-2-4-22(19)40-29(31,32)33/h1-4,9-10,14,16-18H,5-8,11-13H2,(H,37,38)/t16-,17+,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Agonist activity at FXR transfected in human HEK293T cells o-expressing pGL3/hBSEP/luc incubated for 24 hrs by Steady-Glo reagent based luciferase re... |
J Med Chem 63: 3868-3880 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01621
BindingDB Entry DOI: 10.7270/Q2K077QV |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50553986
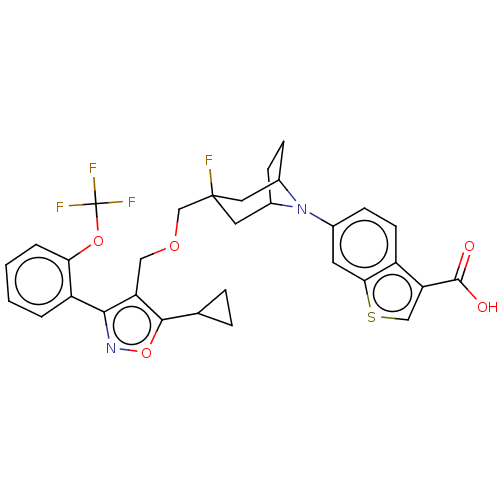
(CHEMBL4794704)Show SMILES OC(=O)c1csc2cc(ccc12)N1C2CCC1CC(F)(COCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C2 |TLB:8:12:18.42.17:14.15| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human FXR expressed in HEK293 cells measured after 18 hrs by steady-glo luciferase reporter gene assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01065
BindingDB Entry DOI: 10.7270/Q2PN9983 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465491
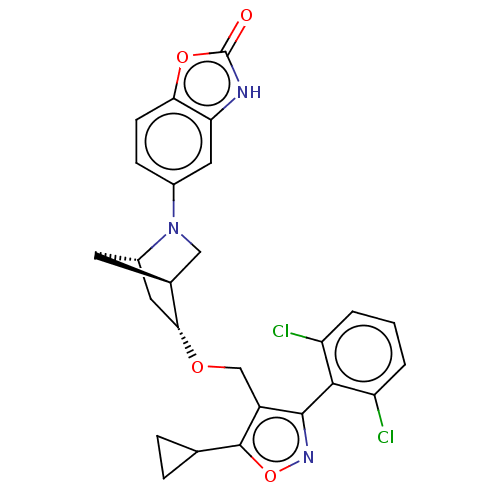
(US10793568, Compound I-140)Show SMILES Clc1cccc(Cl)c1-c1noc(C2CC2)c1CO[C@@H]1C[C@@H]2C[C@H]1CN2c1ccc2oc(=O)[nH]c2c1 |wU:18.20,22.24,wD:20.23,TLB:17:18:23.24:21,25:24:18.19:21,(10.53,-7.71,;10.08,-6.24,;11.13,-5.11,;10.67,-3.64,;9.17,-3.3,;8.12,-4.43,;6.62,-4.08,;8.58,-5.9,;7.53,-7.03,;7.83,-8.54,;6.48,-9.29,;5.35,-8.24,;3.84,-8.54,;2.68,-9.55,;2.38,-8.04,;6,-6.84,;5.25,-5.5,;3.71,-5.48,;2.96,-4.13,;4,-3.19,;2.4,-3.41,;1.35,-6.08,;1.56,-4.54,;.43,-3.24,;1.33,-2.31,;1.33,-.77,;;,1.54,;1.33,2.31,;1.65,3.82,;3.19,3.98,;3.96,5.31,;3.81,2.57,;2.67,1.54,;2.67,,)| Show InChI InChI=1S/C26H23Cl2N3O4/c27-18-2-1-3-19(28)23(18)24-17(25(35-30-24)13-4-5-13)12-33-22-10-16-8-14(22)11-31(16)15-6-7-21-20(9-15)29-26(32)34-21/h1-3,6-7,9,13-14,16,22H,4-5,8,10-12H2,(H,29,32)/t14-,16-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465601
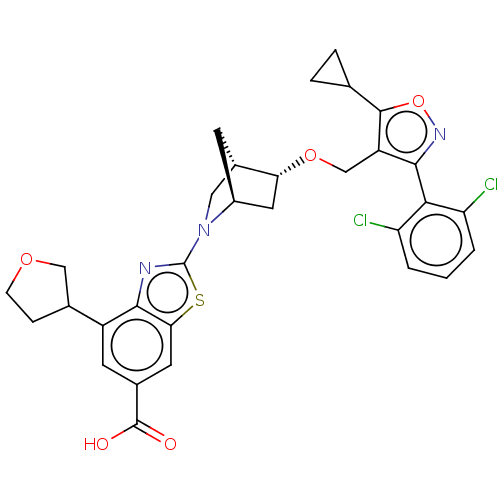
(US10793568, Compound I-251)Show SMILES OC(=O)c1cc(C2CCOC2)c2nc(sc2c1)N1C[C@@H]2C[C@H]1C[C@H]2OCc1c(onc1-c1c(Cl)cccc1Cl)C1CC1 |wU:23.28,19.22,wD:21.23,THB:24:23:18.17:20,13:17:23.22:20,(5.33,1.54,;4,2.31,;4,3.85,;2.67,1.54,;1.33,2.31,;0,1.54,;-1.33,2.31,;-1.49,3.84,;-3,4.16,;-3.77,2.83,;-2.74,1.68,;0,-0,;-1.14,-1.03,;-.52,-2.44,;1.01,-2.28,;1.33,-.77,;2.67,-0,;-1.29,-3.77,;-2.53,-4.12,;-2.21,-5.81,;-3.16,-7.04,;-.91,-5.25,;.59,-5.87,;-.79,-6.16,;-.81,-7.7,;.51,-8.49,;.49,-10.03,;-.77,-10.92,;-.32,-12.39,;1.22,-12.41,;1.72,-10.95,;3.19,-10.5,;4.32,-11.55,;3.98,-13.05,;5.79,-11.09,;6.13,-9.59,;5,-8.55,;3.53,-9,;2.4,-7.95,;-2.23,-10.42,;-3.74,-10.72,;-3.24,-9.26,)| Show InChI InChI=1S/C31H29Cl2N3O5S/c32-22-2-1-3-23(33)26(22)28-21(29(41-35-28)15-4-5-15)14-40-24-11-19-8-18(24)12-36(19)31-34-27-20(16-6-7-39-13-16)9-17(30(37)38)10-25(27)42-31/h1-3,9-10,15-16,18-19,24H,4-8,11-14H2,(H,37,38)/t16?,18-,19-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM465456
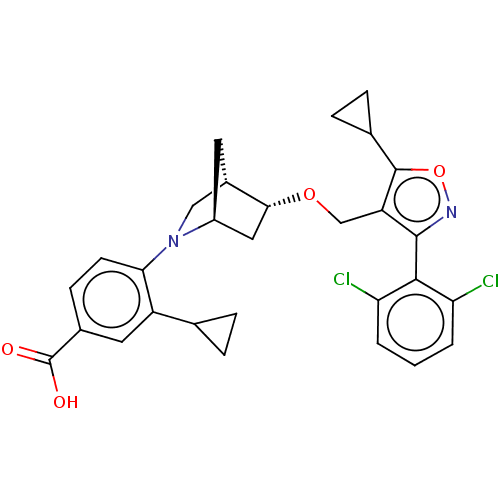
(US10793568, Compound I-108)Show SMILES OC(=O)c1ccc(N2C[C@@H]3C[C@H]2C[C@H]3OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)c(c1)C1CC1 |wU:11.10,13.15,wD:9.9,TLB:14:13:7.8:10,6:7:12.13:10,(-5.11,1.02,;-3.65,1.5,;-3.33,3.01,;-2.5,.47,;-1.04,.95,;.11,-.08,;-.21,-1.58,;.93,-2.61,;.25,-3.85,;1.7,-3.12,;1.83,-.25,;2.13,-1.77,;3.61,-2.64,;3.06,-3.81,;3.56,-5.27,;5.07,-5.56,;5.57,-7.02,;4.69,-8.28,;5.61,-9.51,;7.07,-9.01,;7.05,-7.47,;8.28,-6.54,;9.69,-7.15,;9.88,-8.68,;10.93,-6.22,;10.74,-4.69,;9.32,-4.09,;8.09,-5.02,;6.67,-4.41,;3.15,-8.3,;1.83,-9.09,;1.8,-7.55,;-1.68,-2.06,;-2.82,-1.03,;-1.99,-3.57,;-1.52,-5.03,;-3.02,-4.71,)| Show InChI InChI=1S/C29H28Cl2N2O4/c30-22-2-1-3-23(31)26(22)27-21(28(37-32-27)16-6-7-16)14-36-25-12-19-10-18(25)13-33(19)24-9-8-17(29(34)35)11-20(24)15-4-5-15/h1-3,8-9,11,15-16,18-19,25H,4-7,10,12-14H2,(H,34,35)/t18-,19-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
ARDELYX, INC.
US Patent
| Assay Description
The affinity of FXR ligands for the ligand binding domain of FXR was determined using a commercially available human FXR ligand binding assay (Lantha... |
US Patent US10793568 (2020)
BindingDB Entry DOI: 10.7270/Q21C20Z1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250868

(CHEMBL4083548)Show SMILES OC(=O)c1cc(F)c2nc(sc2c1)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)F)C1CC1 |THB:9:13:15.16:18.19.20,21:19:13:15.16| Show InChI InChI=1S/C29H26F3N3O5S/c30-21-9-15(27(36)37)10-23-25(21)33-29(41-23)35-16-7-8-17(35)12-18(11-16)38-13-20-24(34-40-26(20)14-5-6-14)19-3-1-2-4-22(19)39-28(31)32/h1-4,9-10,14,16-18,28H,5-8,11-13H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in HEK293T cells assessed as BSEP promoter driven cellular transcriptional activity after 24 hrs by luciferas... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50544013
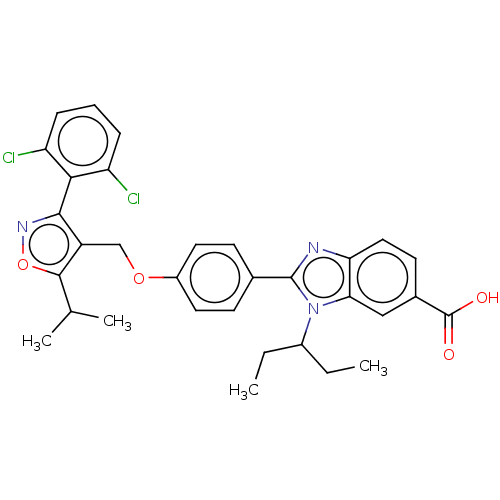
(CHEMBL4638567)Show SMILES CCC(CC)n1c(nc2ccc(cc12)C(O)=O)-c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C(C)C)cc1 |(71.43,-43.38,;72.33,-44.62,;73.86,-44.45,;74.48,-43.05,;73.57,-41.81,;74.77,-45.7,;74.3,-47.17,;75.54,-48.07,;76.78,-47.16,;78.28,-47.47,;79.31,-46.33,;78.83,-44.86,;77.33,-44.56,;76.31,-45.7,;79.85,-43.71,;81.36,-44.03,;79.37,-42.25,;72.83,-47.65,;72.5,-49.16,;71.05,-49.63,;69.9,-48.6,;68.44,-49.07,;67.29,-48.04,;65.82,-48.52,;65.35,-49.99,;63.81,-49.99,;63.33,-48.52,;64.57,-47.61,;64.56,-46.07,;65.9,-45.29,;67.24,-46.06,;65.9,-43.74,;64.56,-42.98,;63.23,-43.75,;63.23,-45.29,;61.9,-46.07,;66.25,-51.23,;65.62,-52.64,;67.78,-51.07,;70.22,-47.1,;71.68,-46.61,)| Show InChI InChI=1S/C32H31Cl2N3O4/c1-5-21(6-2)37-27-16-20(32(38)39)12-15-26(27)35-31(37)19-10-13-22(14-11-19)40-17-23-29(36-41-30(23)18(3)4)28-24(33)8-7-9-25(28)34/h7-16,18,21H,5-6,17H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR expressed in human HuH7 cells by luciferase reporter gene assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115512
BindingDB Entry DOI: 10.7270/Q20G3PQ8 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50250867

(CHEMBL4103787)Show SMILES Cc1cc(cc2sc(nc12)N1C2CCC1CC(C2)OCc1c(onc1-c1ccccc1OC(F)(F)F)C1CC1)C(O)=O |THB:7:10:12.13:15.16.17,18:16:10:12.13| Show InChI InChI=1S/C30H28F3N3O5S/c1-15-10-17(28(37)38)11-24-25(15)34-29(42-24)36-18-8-9-19(36)13-20(12-18)39-14-22-26(35-41-27(22)16-6-7-16)21-4-2-3-5-23(21)40-30(31,32)33/h2-5,10-11,16,18-20H,6-9,12-14H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GST-tagged FXR ligand binding domain (193 to 472 residues) expressed in baculovirus infected insect cells asses... |
J Med Chem 60: 9960-9973 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00907
BindingDB Entry DOI: 10.7270/Q2H997N6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM453881
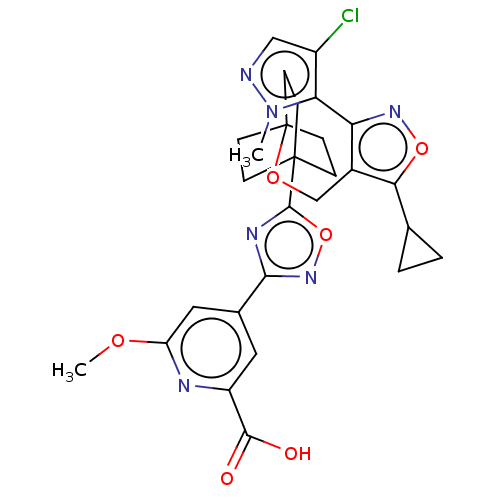
(US10730863, Example 275)Show SMILES COc1cc(cc(n1)C(O)=O)-c1noc(n1)C12CCC(CC1)(CC2)OCc1c(onc1-c1c(Cl)cnn1C)C1CC1 |(9.65,2.62,;8.21,3.17,;7.02,2.2,;5.58,2.75,;4.38,1.78,;4.62,.26,;6.06,-.29,;7.26,.68,;6.3,-1.81,;5.11,-2.78,;7.74,-2.36,;2.95,2.34,;2.55,3.82,;1.01,3.9,;.46,2.47,;1.65,1.5,;-1.03,2.07,;-2.36,.57,;-3.76,.28,;-2.63,1.56,;-.93,1.02,;.48,1.4,;-3.1,2.94,;-1.63,3.4,;-4.17,1.65,;-5.01,.37,;-6.55,.46,;-7.38,1.75,;-8.87,1.36,;-8.96,-.17,;-7.53,-.73,;-7.14,-2.22,;-5.7,-2.78,;-4.41,-1.95,;-5.79,-4.32,;-7.28,-4.71,;-8.12,-3.41,;-9.65,-3.32,;-6.82,3.19,;-5.62,4.15,;-7.05,4.71,)| Show InChI InChI=1S/C28H29ClN6O6/c1-35-22(18(29)13-30-35)21-17(23(40-33-21)15-3-4-15)14-39-28-8-5-27(6-9-28,7-10-28)26-32-24(34-41-26)16-11-19(25(36)37)31-20(12-16)38-2/h11-13,15H,3-10,14H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
A Gal4-hFXR fusion construct reporter system was used as the primary assay to characterize compound activity. A construct including 5 copies of the G... |
US Patent US10730863 (2020)
BindingDB Entry DOI: 10.7270/Q2PR802B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































