 Found 4972 hits of ec50 data for polymerid = 2209,50001093,50004898
Found 4972 hits of ec50 data for polymerid = 2209,50001093,50004898 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349812

(CHEMBL1813010)Show SMILES CCO[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2sccc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C22H25NO5S/c1-4-26-19(22(24)25)13-16-5-7-17(8-6-16)27-11-9-18-15(3)28-21(23-18)20-14(2)10-12-29-20/h5-8,10,12,19H,4,9,11,13H2,1-3H3,(H,24,25)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.000100 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349809

(CHEMBL1813006)Show SMILES CCO[C@@H](Cc1ccc(OCc2nc(oc2C)-c2ccc(C)s2)cc1)C(O)=O |r| Show InChI InChI=1S/C21H23NO5S/c1-4-25-18(21(23)24)11-15-6-8-16(9-7-15)26-12-17-14(3)27-20(22-17)19-10-5-13(2)28-19/h5-10,18H,4,11-12H2,1-3H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.000200 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349808
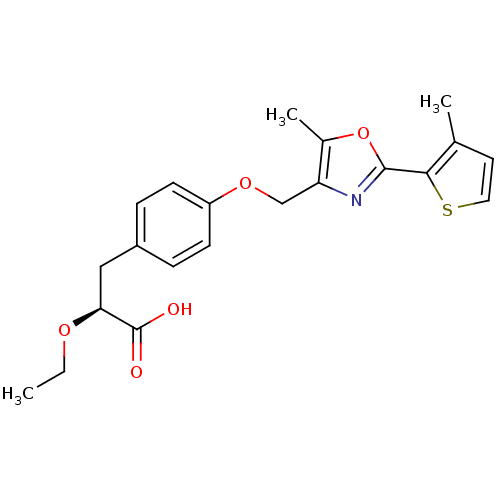
(CHEMBL1813005)Show SMILES CCO[C@@H](Cc1ccc(OCc2nc(oc2C)-c2sccc2C)cc1)C(O)=O |r| Show InChI InChI=1S/C21H23NO5S/c1-4-25-18(21(23)24)11-15-5-7-16(8-6-15)26-12-17-14(3)27-20(22-17)19-13(2)9-10-28-19/h5-10,18H,4,11-12H2,1-3H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.000300 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349807

(CHEMBL1813003)Show SMILES CCO[C@@H](Cc1ccc(OCc2nc(oc2C)-c2cccs2)cc1)C(O)=O |r| Show InChI InChI=1S/C20H21NO5S/c1-3-24-17(20(22)23)11-14-6-8-15(9-7-14)25-12-16-13(2)26-19(21-16)18-5-4-10-27-18/h4-10,17H,3,11-12H2,1-2H3,(H,22,23)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00150 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349813
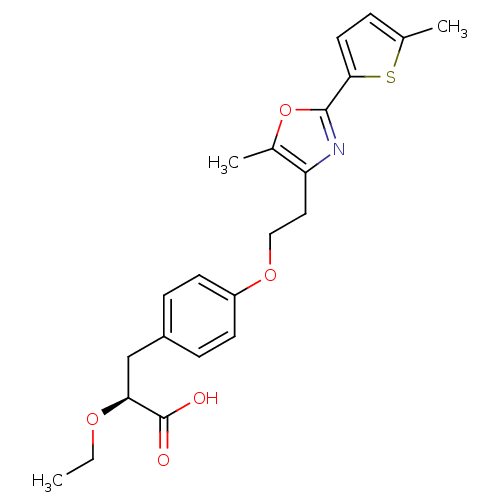
(CHEMBL1813011)Show SMILES CCO[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccc(C)s2)cc1)C(O)=O |r| Show InChI InChI=1S/C22H25NO5S/c1-4-26-19(22(24)25)13-16-6-8-17(9-7-16)27-12-11-18-15(3)28-21(23-18)20-10-5-14(2)29-20/h5-10,19H,4,11-13H2,1-3H3,(H,24,25)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50450236

(Efatutazone)Show SMILES Cc1cc(Oc2ccc3nc(COc4ccc(CC5SC(=O)NC5=O)cc4)n(C)c3c2)cc(C)c1N Show InChI InChI=1S/C27H26N4O4S/c1-15-10-20(11-16(2)25(15)28)35-19-8-9-21-22(13-19)31(3)24(29-21)14-34-18-6-4-17(5-7-18)12-23-26(32)30-27(33)36-23/h4-11,13,23H,12,14,28H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human GAL4-DBD fused PPARgamma LBD expressed in COS7 cells after 24 hrs by luciferase reporter gene assay |
Bioorg Med Chem 26: 5079-5098 (2018)
Article DOI: 10.1016/j.bmc.2018.09.006
BindingDB Entry DOI: 10.7270/Q2J968XC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349811
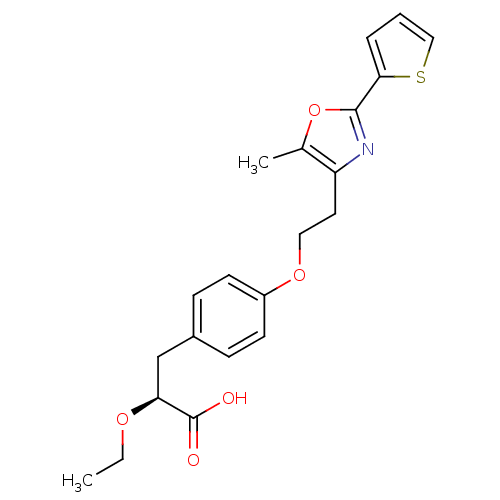
(CHEMBL1813008)Show SMILES CCO[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2cccs2)cc1)C(O)=O |r| Show InChI InChI=1S/C21H23NO5S/c1-3-25-18(21(23)24)13-15-6-8-16(9-7-15)26-11-10-17-14(2)27-20(22-17)19-5-4-12-28-19/h4-9,12,18H,3,10-11,13H2,1-2H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471968
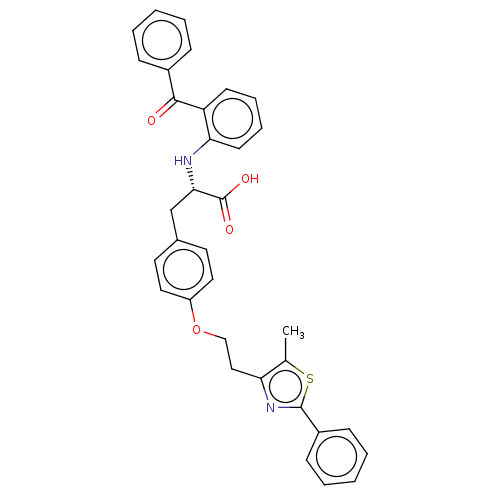
(CHEMBL358379)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O4S/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARgamma assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471968

(CHEMBL358379)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O4S/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50004753
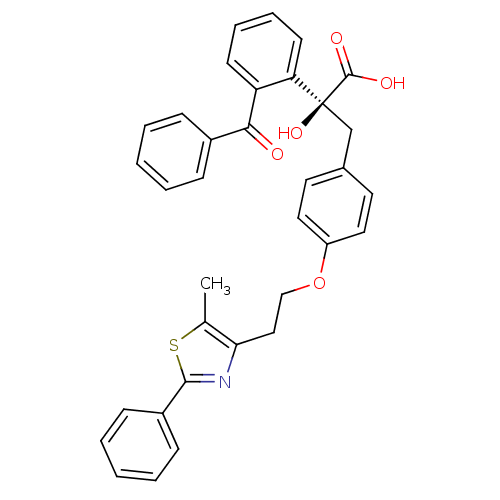
(CHEMBL2282517)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@@](O)(C(O)=O)c2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H29NO5S/c1-23-30(35-32(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-34(39,33(37)38)29-15-9-8-14-28(29)31(36)25-10-4-2-5-11-25/h2-19,39H,20-22H2,1H3,(H,37,38)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50004753

(CHEMBL2282517)Show SMILES Cc1sc(nc1CCOc1ccc(C[C@@](O)(C(O)=O)c2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H29NO5S/c1-23-30(35-32(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-34(39,33(37)38)29-15-9-8-14-28(29)31(36)25-10-4-2-5-11-25/h2-19,39H,20-22H2,1H3,(H,37,38)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28783

((3E)-3-(ethoxyimino)-2-({4-[2-(5-methyl-2-phenyl-1...)Show SMILES CCO\N=C(/CC)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H30N2O5/c1-4-23(28-32-5-2)22(26(29)30)17-19-11-13-21(14-12-19)31-16-15-24-18(3)33-25(27-24)20-9-7-6-8-10-20/h6-14,22H,4-5,15-17H2,1-3H3,(H,29,30)/b28-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471982
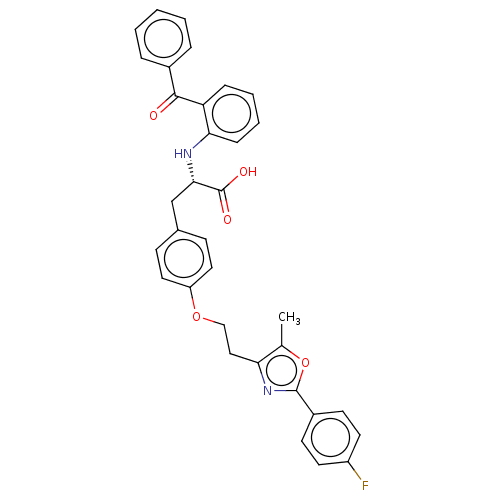
(CHEMBL147384)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FN2O5/c1-22-29(37-33(42-22)25-13-15-26(35)16-14-25)19-20-41-27-17-11-23(12-18-27)21-31(34(39)40)36-30-10-6-5-9-28(30)32(38)24-7-3-2-4-8-24/h2-18,31,36H,19-21H2,1H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471982

(CHEMBL147384)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H29FN2O5/c1-22-29(37-33(42-22)25-13-15-26(35)16-14-25)19-20-41-27-17-11-23(12-18-27)21-31(34(39)40)36-30-10-6-5-9-28(30)32(38)24-7-3-2-4-8-24/h2-18,31,36H,19-21H2,1H3,(H,39,40)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARgamma assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28780
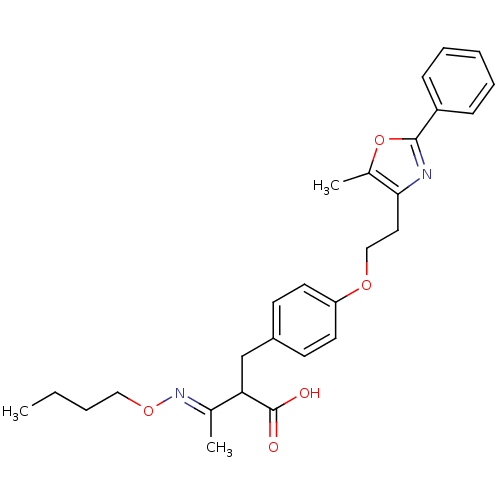
((3E)-3-(butoxyimino)-2-({4-[2-(5-methyl-2-phenyl-1...)Show SMILES CCCCO\N=C(/C)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H32N2O5/c1-4-5-16-33-29-19(2)24(27(30)31)18-21-11-13-23(14-12-21)32-17-15-25-20(3)34-26(28-25)22-9-7-6-8-10-22/h6-14,24H,4-5,15-18H2,1-3H3,(H,30,31)/b29-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50151001
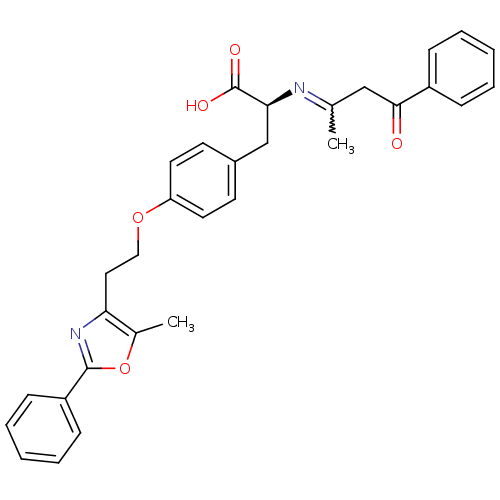
((S)-2-((Z)-1-Methyl-3-oxo-3-phenyl-propenylamino)-...)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O |w:1.0| Show InChI InChI=1S/C31H30N2O5/c1-21(19-29(34)24-9-5-3-6-10-24)32-28(31(35)36)20-23-13-15-26(16-14-23)37-18-17-27-22(2)38-30(33-27)25-11-7-4-8-12-25/h3-16,28H,17-20H2,1-2H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Effective concentration against human peroxisome proliferator activated receptor gamma in Gal4 transactivation assay |
J Med Chem 47: 4118-27 (2004)
Article DOI: 10.1021/jm030631e
BindingDB Entry DOI: 10.7270/Q2DR2TZ5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471980
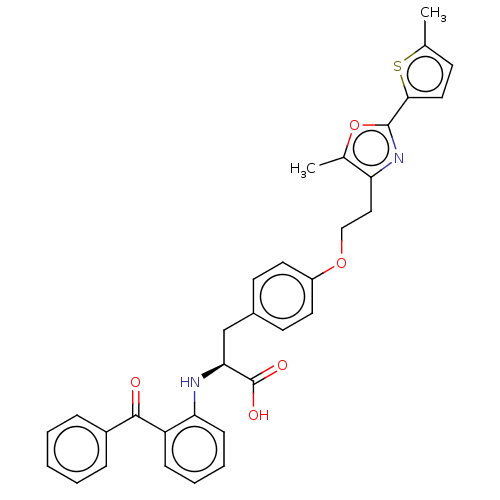
(CHEMBL147095)Show SMILES Cc1ccc(s1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C33H30N2O5S/c1-21-12-17-30(41-21)32-35-27(22(2)40-32)18-19-39-25-15-13-23(14-16-25)20-29(33(37)38)34-28-11-7-6-10-26(28)31(36)24-8-4-3-5-9-24/h3-17,29,34H,18-20H2,1-2H3,(H,37,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.245 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50013727

(CHEBI:79990 | CHEMBL410478 | GW409544)Show SMILES C\C(N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O)=C\C(=O)c1ccccc1 |r| Show InChI InChI=1S/C31H30N2O5/c1-21(19-29(34)24-9-5-3-6-10-24)32-28(31(35)36)20-23-13-15-26(16-14-23)37-18-17-27-22(2)38-30(33-27)25-11-7-4-8-12-25/h3-16,19,28,32H,17-18,20H2,1-2H3,(H,35,36)/b21-19-/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
University of Oklahoma
Curated by ChEMBL
| Assay Description
Agonist activity at GAL4-fused human PPARgamma LBD |
Bioorg Med Chem Lett 28: 2717-2722 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.010
BindingDB Entry DOI: 10.7270/Q24F1TCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50151001

((S)-2-((Z)-1-Methyl-3-oxo-3-phenyl-propenylamino)-...)Show SMILES CC(CC(=O)c1ccccc1)=N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O |w:1.0| Show InChI InChI=1S/C31H30N2O5/c1-21(19-29(34)24-9-5-3-6-10-24)32-28(31(35)36)20-23-13-15-26(16-14-23)37-18-17-27-22(2)38-30(33-27)25-11-7-4-8-12-25/h3-16,28H,17-20H2,1-2H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
USDA-ARS
Curated by ChEMBL
| Assay Description
Agonist activity at human PPARgamma |
Bioorg Med Chem 16: 3800-8 (2008)
Article DOI: 10.1016/j.bmc.2008.01.051
BindingDB Entry DOI: 10.7270/Q2WW7JKJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472035
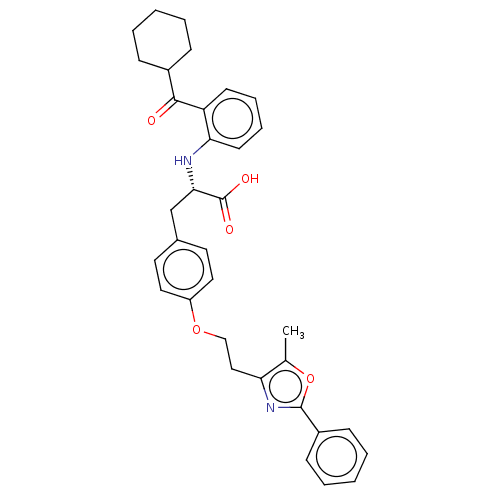
(CHEMBL2112870)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)C2CCCCC2)C(O)=O)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H36N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h3,6-9,12-19,25,31,35H,2,4-5,10-11,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.282 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
in vitro agonist activity against peroxisome proliferator activated receptor-gamma (PPAR-gamma), using alkaline phosphatase activity transactivator a... |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28779

((3E)-2-({4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)e...)Show SMILES CC(C)CO\N=C(/C)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H32N2O5/c1-18(2)17-33-29-19(3)24(27(30)31)16-21-10-12-23(13-11-21)32-15-14-25-20(4)34-26(28-25)22-8-6-5-7-9-22/h5-13,18,24H,14-17H2,1-4H3,(H,30,31)/b29-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472041

(CHEMBL147770)Show SMILES CCCOC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-3-18-38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)20-22-13-15-24(16-14-22)37-19-17-26-21(2)39-29(33-26)23-9-5-4-6-10-23/h4-16,28,32H,3,17-20H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
in vitro agonist activity against peroxisome proliferator activated receptor-gamma (PPAR-gamma), using alkaline phosphatase activity transactivator a... |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28782

((3E)-3-(methoxyimino)-2-({4-[2-(5-methyl-2-phenyl-...)Show SMILES CC\C(=N/OC)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H28N2O5/c1-4-22(27-30-3)21(25(28)29)16-18-10-12-20(13-11-18)31-15-14-23-17(2)32-24(26-23)19-8-6-5-7-9-19/h5-13,21H,4,14-16H2,1-3H3,(H,28,29)/b27-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| n/a | n/a | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARgamma assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
in vitro agonist activity against peroxisome proliferator activated receptor-gamma (PPAR-gamma), using alkaline phosphatase activity transactivator a... |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Transactivation of PPARgamma assessed as induction of alkaline phosphatase activity |
Bioorg Med Chem Lett 20: 3344-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.031
BindingDB Entry DOI: 10.7270/Q2C53N33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research & Development
Curated by ChEMBL
| Assay Description
Agonist activity for Human PPAR gamma receptor in transcriptional activation assay |
J Med Chem 43: 527-50 (2000)
BindingDB Entry DOI: 10.7270/Q2H994DT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50004754

(CHEMBL2282523)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](O)(C(O)=O)c2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H29NO6/c1-23-30(35-32(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-34(39,33(37)38)29-15-9-8-14-28(29)31(36)25-10-4-2-5-11-25/h2-19,39H,20-22H2,1H3,(H,37,38)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Maximal reporter activity against human Peroxisome proliferator activated receptor gamma Gal4 chimeric in transiently transfected CV-1 cells by funct... |
Bioorg Med Chem Lett 11: 3111-3 (2001)
BindingDB Entry DOI: 10.7270/Q2B27TKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50004754

(CHEMBL2282523)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@](O)(C(O)=O)c2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 |r| Show InChI InChI=1S/C34H29NO6/c1-23-30(35-32(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-34(39,33(37)38)29-15-9-8-14-28(29)31(36)25-10-4-2-5-11-25/h2-19,39H,20-22H2,1H3,(H,37,38)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... |
Citation and Details
Article DOI: 10.1007/s00044-012-0003-4
BindingDB Entry DOI: 10.7270/Q280544F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Activation of peroxisome proliferator activated receptor gamma measured by induction of 50% of maximum alkaline phosphatase activity, transfection as... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research & Development
Curated by ChEMBL
| Assay Description
Agonist activity for murine PPAR gamma receptor in transcriptional activation assay |
J Med Chem 43: 527-50 (2000)
BindingDB Entry DOI: 10.7270/Q2H994DT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was tested functionally in vitro for inducing 50% of the maximum alkaline phosphatase activity (Transactivation) against murine Peroxisome p... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28781
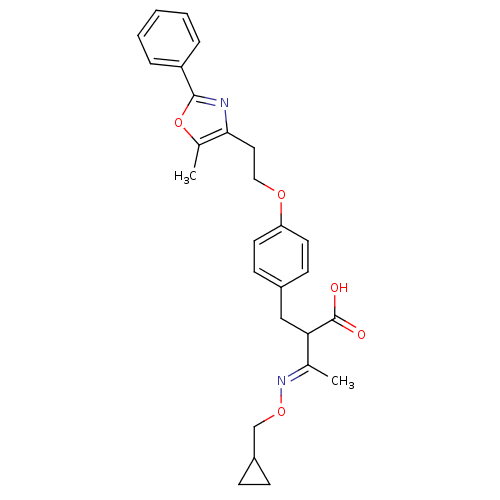
((3E)-3-[(cyclopropylmethoxy)imino]-2-({4-[2-(5-met...)Show SMILES C\C(=N/OCC1CC1)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H30N2O5/c1-18(29-33-17-21-8-9-21)24(27(30)31)16-20-10-12-23(13-11-20)32-15-14-25-19(2)34-26(28-25)22-6-4-3-5-7-22/h3-7,10-13,21,24H,8-9,14-17H2,1-2H3,(H,30,31)/b29-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50093472

(CHEMBL3585575)Show SMILES CC[C@@H](OC(=O)N(Cc1ccccc1)C(=O)Nc1cc(OC)cc(OC)c1)c1cc(no1)-c1ccc(OC(C)(C)C(O)=O)cc1 |r| Show InChI InChI=1S/C23H32N12O8/c24-22-30-16(10-18(32-22)34(6-28-10)20-14(40)12(38)8(4-36)42-20)26-2-1-3-27-17-11-19(33-23(25)31-17)35(7-29-11)21-15(41)13(39)9(5-37)43-21/h6-9,12-15,20-21,36-41H,1-5H2,(H3,24,26,30,32)(H3,25,27,31,33)/t8-,9+,12+,13-,14?,15?,20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Universitat Rovira i Virgili (URV)
Curated by ChEMBL
| Assay Description
Activity at PPARgamma (unknown origin) assessed as transactivation activity by reporter gene assay |
J Med Chem 58: 5381-94 (2015)
Article DOI: 10.1021/jm501155f
BindingDB Entry DOI: 10.7270/Q2K07601 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50093472

(CHEMBL3585575)Show SMILES CC[C@@H](OC(=O)N(Cc1ccccc1)C(=O)Nc1cc(OC)cc(OC)c1)c1cc(no1)-c1ccc(OC(C)(C)C(O)=O)cc1 |r| Show InChI InChI=1S/C23H32N12O8/c24-22-30-16(10-18(32-22)34(6-28-10)20-14(40)12(38)8(4-36)42-20)26-2-1-3-27-17-11-19(33-23(25)31-17)35(7-29-11)21-15(41)13(39)9(5-37)43-21/h6-9,12-15,20-21,36-41H,1-5H2,(H3,24,26,30,32)(H3,25,27,31,33)/t8-,9+,12+,13-,14?,15?,20-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Universit£ Degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Transactivation of PPARgamma (unknown origin) |
Eur J Med Chem 176: 326-342 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.012
BindingDB Entry DOI: 10.7270/Q2HQ43BR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50135450
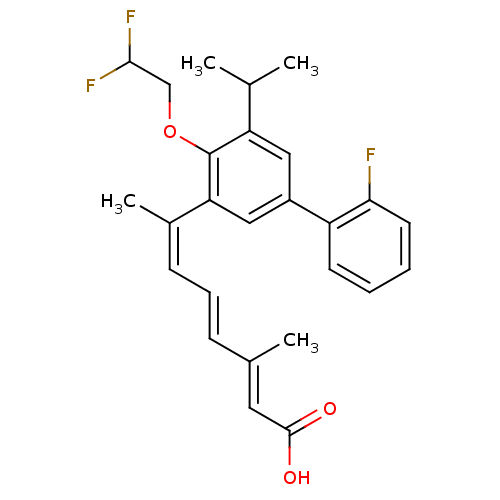
((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-2'-fluoro-5-...)Show SMILES CC(C)c1cc(cc(\C(C)=C/C=C/C(/C)=C/C(O)=O)c1OCC(F)F)-c1ccccc1F Show InChI InChI=1S/C26H27F3O3/c1-16(2)21-13-19(20-10-5-6-11-23(20)27)14-22(26(21)32-15-24(28)29)18(4)9-7-8-17(3)12-25(30)31/h5-14,16,24H,15H2,1-4H3,(H,30,31)/b8-7+,17-12+,18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
In vitro agonistic activity against RXR alpha in CV-1 cells |
Bioorg Med Chem Lett 13: 4071-5 (2003)
BindingDB Entry DOI: 10.7270/Q2V987GP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50557786

(CHEMBL4776913)Show SMILES Cn1c(COc2ccc(CC3SC(=O)NC3=O)cc2)nc2ccc(Oc3ccc(O)c(c3)C(C)(C)C)cc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at full-length PPARgamma (unknown origin) expressed in MG63 cells assessed as transcriptional activation incubated for 18 hrs by dua... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00100
BindingDB Entry DOI: 10.7270/Q25H7KZK |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472011

(CHEMBL148300)Show SMILES CCOC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C30H30N2O6/c1-3-36-30(35)24-11-7-8-12-26(24)31-27(29(33)34)19-21-13-15-23(16-14-21)37-18-17-25-20(2)38-28(32-25)22-9-5-4-6-10-22/h4-16,27,31H,3,17-19H2,1-2H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
-log concentration required to induce 50% maximum lipogenic activity against Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50106109

(4-Isocyanato-benzoic acid 2-((2,4-bis-trifluoromet...)Show SMILES CC(C)(Oc1cccc(CCCN(CCOC(=O)c2ccc(cc2)N=C=O)Cc2ccc(cc2C(F)(F)F)C(F)(F)F)c1)C(O)=O Show InChI InChI=1S/C32H30F6N2O6/c1-30(2,29(43)44)46-26-7-3-5-21(17-26)6-4-14-40(15-16-45-28(42)22-9-12-25(13-10-22)39-20-41)19-23-8-11-24(31(33,34)35)18-27(23)32(36,37)38/h3,5,7-13,17-18H,4,6,14-16,19H2,1-2H3,(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonistic transcriptional activity in CV-1 cells expressing Gal4-PPAR gamma chimera |
Bioorg Med Chem Lett 11: 2959-62 (2001)
BindingDB Entry DOI: 10.7270/Q2V69HWQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28784
((3E)-3-{[(4-fluorophenyl)methoxy]imino}-2-({4-[2-(...)Show SMILES C\C(=N/OCc1ccc(F)cc1)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C30H29FN2O5/c1-20(33-37-19-23-8-12-25(31)13-9-23)27(30(34)35)18-22-10-14-26(15-11-22)36-17-16-28-21(2)38-29(32-28)24-6-4-3-5-7-24/h3-15,27H,16-19H2,1-2H3,(H,34,35)/b33-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28778
((3E)-2-({4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)e...)Show SMILES CCCO\N=C(/C)C(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H30N2O5/c1-4-15-32-28-18(2)23(26(29)30)17-20-10-12-22(13-11-20)31-16-14-24-19(3)33-25(27-24)21-8-6-5-7-9-21/h5-13,23H,4,14-17H2,1-3H3,(H,29,30)/b28-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a |
LG Life Sciences
| Assay Description
The HepG2 cells were co-transfected with DNA construct containing PPAR-Gal4 chimeric receptor and Gal4-luciferase reporter. EC50 is the concentration... |
Bioorg Med Chem Lett 17: 937-41 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.050
BindingDB Entry DOI: 10.7270/Q2057D8J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50472019
(CHEMBL148668)Show SMILES CC(C)OC(=O)c1ccccc1NC(Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C31H32N2O6/c1-20(2)38-31(36)25-11-7-8-12-27(25)32-28(30(34)35)19-22-13-15-24(16-14-22)37-18-17-26-21(3)39-29(33-26)23-9-5-4-6-10-23/h4-16,20,28,32H,17-19H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.575 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
in vitro agonist activity against peroxisome proliferator activated receptor-gamma (PPAR-gamma), using alkaline phosphatase activity transactivator a... |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM614895
(A7-54 rac-2-((3R,4R)-4-(((6- (cyclopropyl(4-(1,1,1...)Show SMILES NC(=O)CN1CC[C@@H](CNc2ncnc(N(Cc3ccc(cc3)C(O)(C(F)(F)F)C(F)(F)F)C3CC3)c2F)[C@H](O)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc.
US Patent
| |
US Patent US10173996 (2019)
BindingDB Entry DOI: 10.7270/Q2TT4W2H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma receptor expressed in HEK293 cells by GAL4 transactivation assay |
Bioorg Med Chem Lett 17: 2312-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.060
BindingDB Entry DOI: 10.7270/Q21N80ST |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50349816

(CHEMBL1813009)Show SMILES CCO[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccsc2)cc1)C(O)=O |r| Show InChI InChI=1S/C21H23NO5S/c1-3-25-19(21(23)24)12-15-4-6-17(7-5-15)26-10-8-18-14(2)27-20(22-18)16-9-11-28-13-16/h4-7,9,11,13,19H,3,8,10,12H2,1-2H3,(H,23,24)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Transactivation of human PPARgamma expressed in human HepG2 cells cotransfected with PPRE3-TK-Luc by luciferase reporter gene assay |
Bioorg Med Chem Lett 21: 3103-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.020
BindingDB Entry DOI: 10.7270/Q2ZC837H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471953
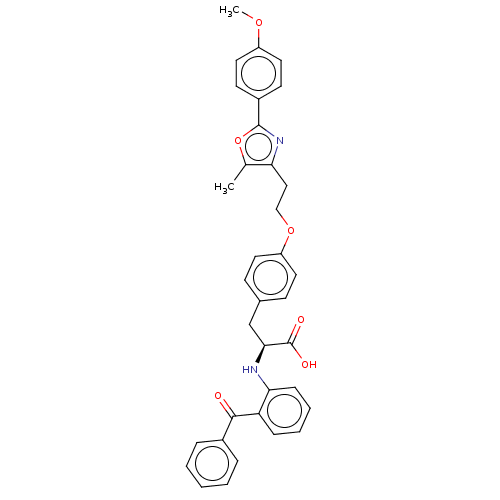
(CHEMBL356382)Show SMILES COc1ccc(cc1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)26-14-18-27(41-2)19-15-26)20-21-42-28-16-12-24(13-17-28)22-32(35(39)40)36-31-11-7-6-10-29(31)33(38)25-8-4-3-5-9-25/h3-19,32,36H,20-22H2,1-2H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Tested functionally in vitro for inducing 50% of the maximum alkaline phosphate activity (Transactivation) against Peroxisome proliferator activated ... |
J Med Chem 41: 5037-54 (1998)
Article DOI: 10.1021/jm980413z
BindingDB Entry DOI: 10.7270/Q2J38W9F |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50471953

(CHEMBL356382)Show SMILES COc1ccc(cc1)-c1nc(CCOc2ccc(C[C@H](Nc3ccccc3C(=O)c3ccccc3)C(O)=O)cc2)c(C)o1 Show InChI InChI=1S/C35H32N2O6/c1-23-30(37-34(43-23)26-14-18-27(41-2)19-15-26)20-21-42-28-16-12-24(13-17-28)22-32(35(39)40)36-31-11-7-6-10-29(31)33(38)25-8-4-3-5-9-25/h3-19,32,36H,20-22H2,1-2H3,(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Transactivation of Homo sapiens (human) PPARgamma assessed as luciferase activity by reporter gene assay |
Citation and Details
Article DOI: 10.1007/s00044-011-9818-7
BindingDB Entry DOI: 10.7270/Q2R49TNP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085046
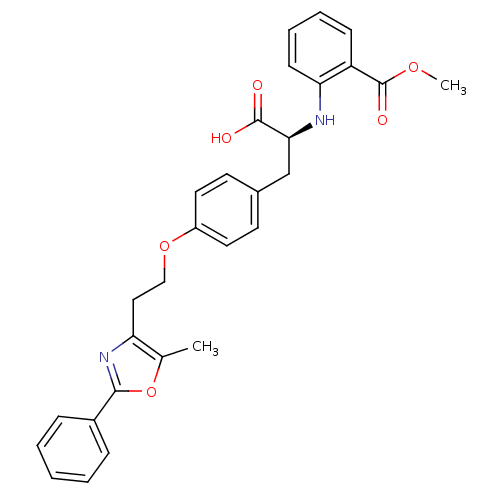
(2-((S)-1-carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol...)Show SMILES COC(=O)c1ccccc1N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C29H28N2O6/c1-19-24(31-27(37-19)21-8-4-3-5-9-21)16-17-36-22-14-12-20(13-15-22)18-26(28(32)33)30-25-11-7-6-10-23(25)29(34)35-2/h3-15,26,30H,16-18H2,1-2H3,(H,32,33)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.617 | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
-log concentration required to induce 50% maximum lipogenic activity against Peroxisome proliferator activated receptor gamma |
J Med Chem 41: 5055-69 (1998)
Article DOI: 10.1021/jm980414r
BindingDB Entry DOI: 10.7270/Q2DB84MJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data