 Found 678 hits of ec50 data for polymerid = 2411
Found 678 hits of ec50 data for polymerid = 2411 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384640
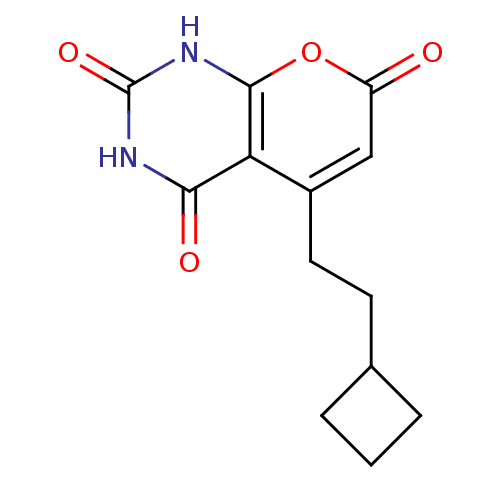
(CHEMBL2036954)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(5-4-7-2-1-3-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384612
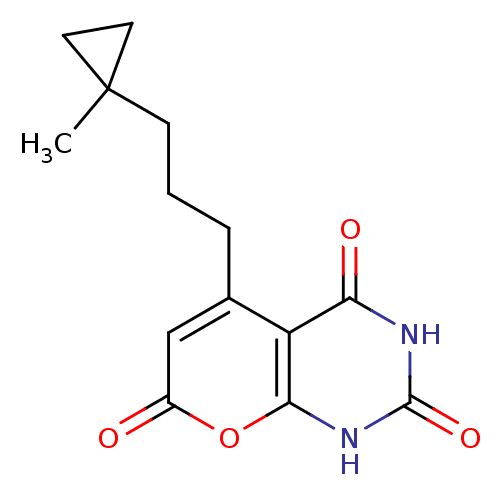
(CHEMBL2036958)Show SMILES CC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C14H16N2O4/c1-14(5-6-14)4-2-3-8-7-9(17)20-12-10(8)11(18)15-13(19)16-12/h7H,2-6H2,1H3,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384637
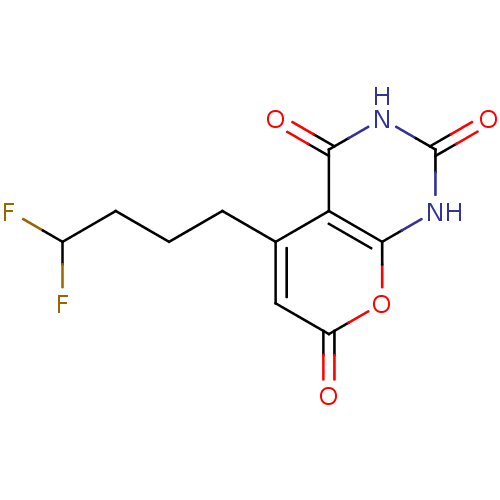
(CHEMBL2036951)Show InChI InChI=1S/C11H10F2N2O4/c12-6(13)3-1-2-5-4-7(16)19-10-8(5)9(17)14-11(18)15-10/h4,6H,1-3H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384634

(CHEMBL2036948)Show InChI InChI=1S/C12H14N2O4/c1-6(2)3-4-7-5-8(15)18-11-9(7)10(16)13-12(17)14-11/h5-6H,3-4H2,1-2H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384641
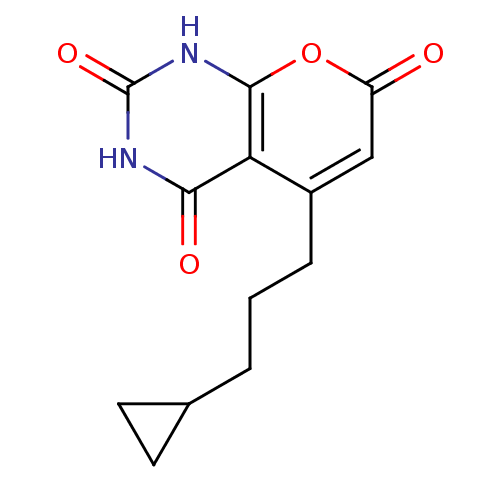
(CHEMBL2036955)Show InChI InChI=1S/C13H14N2O4/c16-9-6-8(3-1-2-7-4-5-7)10-11(17)14-13(18)15-12(10)19-9/h6-7H,1-5H2,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384636

(CHEMBL2036950)Show InChI InChI=1S/C13H16N2O4/c1-7(2)4-3-5-8-6-9(16)19-12-10(8)11(17)14-13(18)15-12/h6-7H,3-5H2,1-2H3,(H2,14,15,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23525
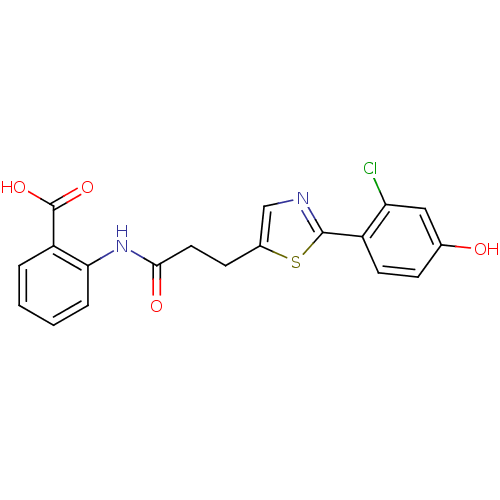
(2-{3-[2-(2-chloro-4-hydroxyphenyl)-1,3-thiazol-5-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnc(s1)-c1ccc(O)cc1Cl Show InChI InChI=1S/C19H15ClN2O4S/c20-15-9-11(23)5-7-13(15)18-21-10-12(27-18)6-8-17(24)22-16-4-2-1-3-14(16)19(25)26/h1-5,7,9-10,23H,6,8H2,(H,22,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109A receptor expressed in CHO-K1 cells after 60 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2721-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.116
BindingDB Entry DOI: 10.7270/Q2N29X8C |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50319112
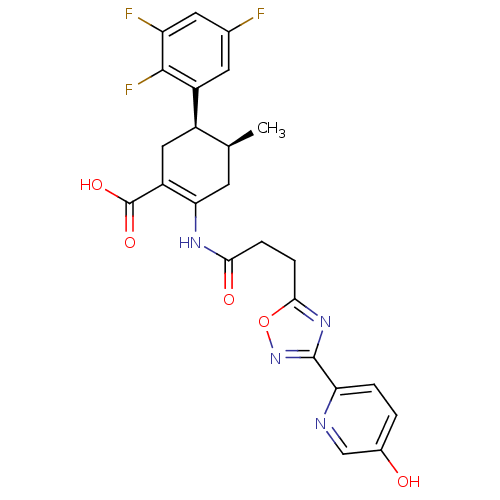
((4S,5R)-2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxad...)Show SMILES C[C@H]1CC(NC(=O)CCc2nc(no2)-c2ccc(O)cn2)=C(C[C@H]1c1cc(F)cc(F)c1F)C(O)=O |r,c:22| Show InChI InChI=1S/C24H21F3N4O5/c1-11-6-19(16(24(34)35)9-14(11)15-7-12(25)8-17(26)22(15)27)29-20(33)4-5-21-30-23(31-36-21)18-3-2-13(32)10-28-18/h2-3,7-8,10-11,14,32H,4-6,9H2,1H3,(H,29,33)(H,34,35)/t11-,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23525

(2-{3-[2-(2-chloro-4-hydroxyphenyl)-1,3-thiazol-5-y...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1cnc(s1)-c1ccc(O)cc1Cl Show InChI InChI=1S/C19H15ClN2O4S/c20-15-9-11(23)5-7-13(15)18-21-10-12(27-18)6-8-17(24)22-16-4-2-1-3-14(16)19(25)26/h1-5,7,9-10,23H,6,8H2,(H,22,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | 6 | n/a | n/a | 7.4 | 23 |
Merck Research Laboratories
| Assay Description
Membranes were incubated in binding buffer with [5, 6-3H]-niacin in the presence of test compound. After 4 hours at room temperature, reactions were ... |
J Med Chem 50: 6303-6 (2007)
Article DOI: 10.1021/jm700942d
BindingDB Entry DOI: 10.7270/Q29S1PBG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277715
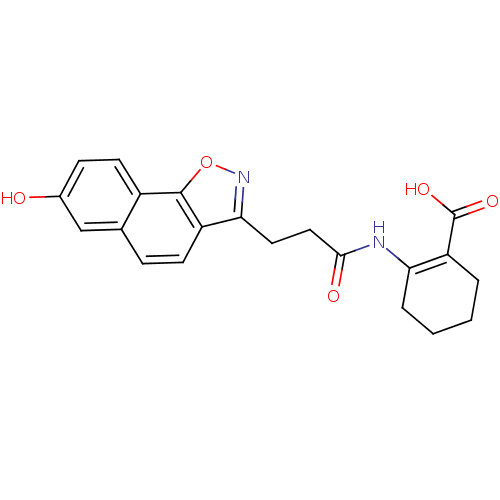
(2-(3-(7-hydroxynaphtho[2,1-d]isoxazol-3-yl)propana...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1cc(O)ccc21 |t:3| Show InChI InChI=1S/C21H20N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h5-8,11,24H,1-4,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384644
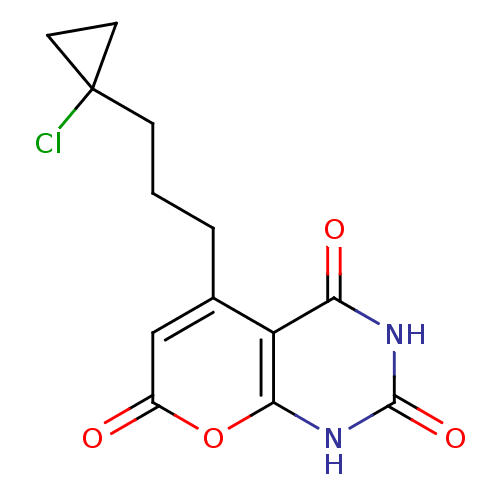
(CHEMBL2036960)Show SMILES ClC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C13H13ClN2O4/c14-13(4-5-13)3-1-2-7-6-8(17)20-11-9(7)10(18)15-12(19)16-11/h6H,1-5H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in human adipocytes assessed as decrease in intracellular cAMP level by HTRF assay |
Bioorg Med Chem Lett 17: 5620-3 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.07.101
BindingDB Entry DOI: 10.7270/Q2TT4QN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50268973

(CHEMBL4082765)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCC#Cc1ccc(O)cc1Cl |t:3| Show InChI InChI=1S/C18H18ClNO4/c19-15-11-13(21)10-9-12(15)5-1-4-8-17(22)20-16-7-3-2-6-14(16)18(23)24/h9-11,21H,2-4,6-8H2,(H,20,22)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human HCA2 receptor expressed in Flp-IN HEK cells assessed as reduction in forskolin-stimulated cAMP accumulation mea... |
Bioorg Med Chem 25: 4314-4329 (2017)
Article DOI: 10.1016/j.bmc.2017.06.028
BindingDB Entry DOI: 10.7270/Q2D22140 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384638

(CHEMBL2036952)Show SMILES FC(F)(F)CCCc1cc(=O)oc2[nH]c(=O)[nH]c(=O)c12 Show InChI InChI=1S/C11H9F3N2O4/c12-11(13,14)3-1-2-5-4-6(17)20-9-7(5)8(18)15-10(19)16-9/h4H,1-3H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384643
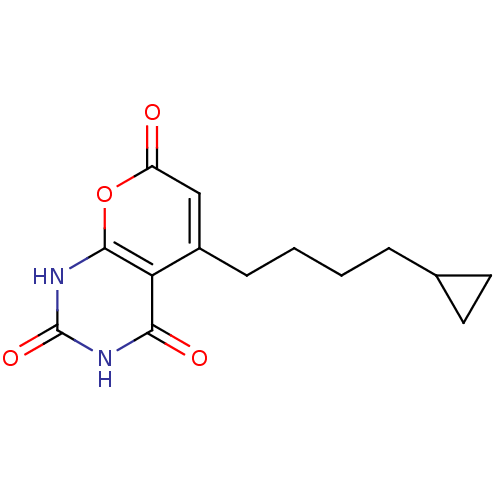
(CHEMBL2036957)Show InChI InChI=1S/C14H16N2O4/c17-10-7-9(4-2-1-3-8-5-6-8)11-12(18)15-14(19)16-13(11)20-10/h7-8H,1-6H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50319095
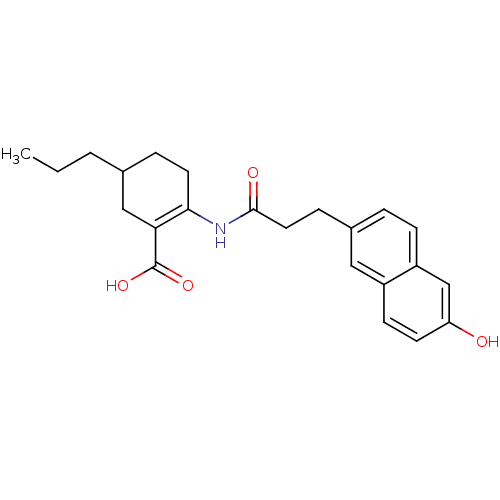
(2-(3-(6-hydroxynaphthalen-2-yl)propanamido)-5-prop...)Show SMILES CCCC1CCC(NC(=O)CCc2ccc3cc(O)ccc3c2)=C(C1)C(O)=O |c:24| Show InChI InChI=1S/C23H27NO4/c1-2-3-15-5-10-21(20(13-15)23(27)28)24-22(26)11-6-16-4-7-18-14-19(25)9-8-17(18)12-16/h4,7-9,12,14-15,25H,2-3,5-6,10-11,13H2,1H3,(H,24,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50405697
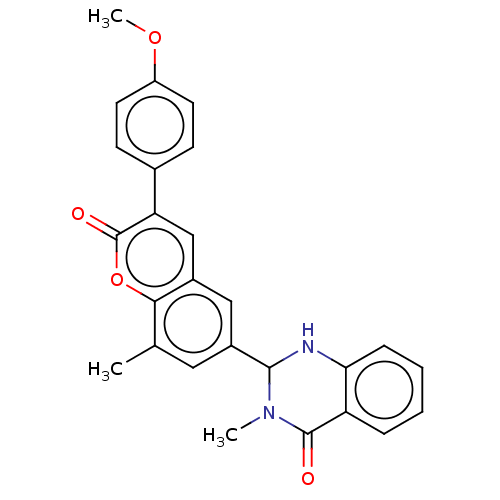
(CHEMBL4164140)Show SMILES COc1ccc(cc1)-c1cc2cc(cc(C)c2oc1=O)C1Nc2ccccc2C(=O)N1C Show InChI InChI=1S/C15H25NO3S/c1-10(9-20)14(17)16-8-12(7-13(16)15(18)19)11-5-3-2-4-6-11/h10-13,20H,2-9H2,1H3,(H,18,19)/t10-,12+,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in HEK293 cells harboring glosensor-22F cAMP plasmid DNA assessed as inhibition of forskolin-stimulated c... |
Eur J Med Chem 152: 208-222 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.037
BindingDB Entry DOI: 10.7270/Q2B27XT0 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50405697

(CHEMBL4164140)Show SMILES COc1ccc(cc1)-c1cc2cc(cc(C)c2oc1=O)C1Nc2ccccc2C(=O)N1C Show InChI InChI=1S/C15H25NO3S/c1-10(9-20)14(17)16-8-12(7-13(16)15(18)19)11-5-3-2-4-6-11/h10-13,20H,2-9H2,1H3,(H,18,19)/t10-,12+,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in HEK293 cells harboring glosensor-22F cAMP plasmid DNA assessed as inhibition of forskolin-stimulated c... |
Eur J Med Chem 152: 208-222 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.037
BindingDB Entry DOI: 10.7270/Q2B27XT0 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384630
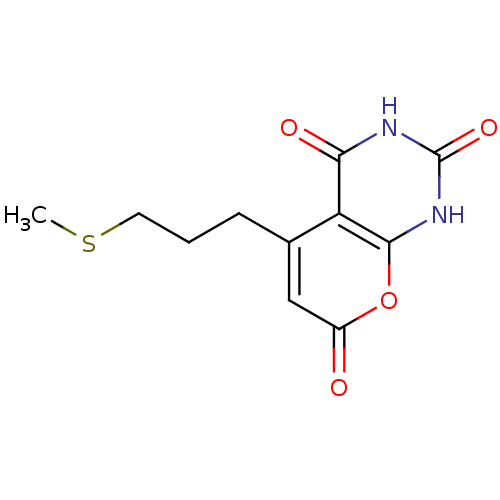
(CHEMBL2036828)Show InChI InChI=1S/C11H12N2O4S/c1-18-4-2-3-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50319102

(5-(3,5-difluorophenyl)-2-(3-(6-hydroxynaphthalen-2...)Show SMILES OC(=O)C1=C(CCC(C1)c1cc(F)cc(F)c1)NC(=O)CCc1ccc2cc(O)ccc2c1 |t:3| Show InChI InChI=1S/C26H23F2NO4/c27-20-10-19(11-21(28)14-20)18-5-7-24(23(13-18)26(32)33)29-25(31)8-2-15-1-3-17-12-22(30)6-4-16(17)9-15/h1,3-4,6,9-12,14,18,30H,2,5,7-8,13H2,(H,29,31)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50388230

(CHEMBL2057618)Show InChI InChI=1S/C8H8N2O2/c11-8(12)7-5-2-3-1-4(3)6(5)9-10-7/h3-4H,1-2H2,(H,9,10)(H,11,12)/t3-,4-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in CHO cells assessed as decrease in forskolin-stimulated cAMP production by HTRF assay |
J Med Chem 55: 3644-66 (2012)
Article DOI: 10.1021/jm2010964
BindingDB Entry DOI: 10.7270/Q2000353 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384639
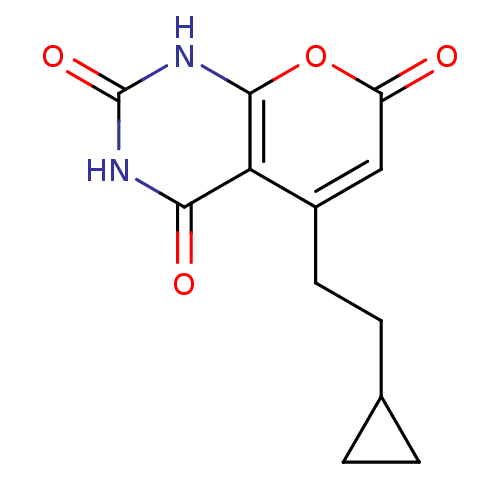
(CHEMBL2036953)Show InChI InChI=1S/C12H12N2O4/c15-8-5-7(4-3-6-1-2-6)9-10(16)13-12(17)14-11(9)18-8/h5-6H,1-4H2,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50388228
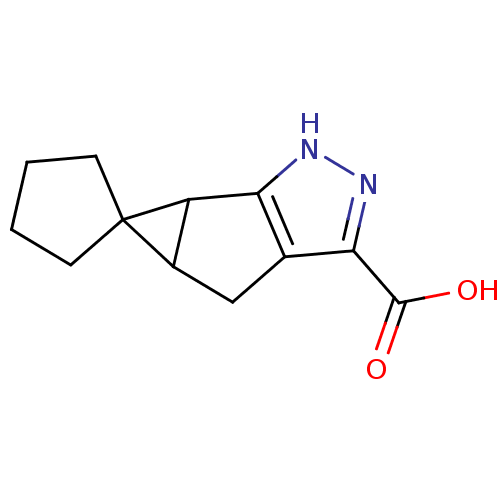
(CHEMBL2057610)Show InChI InChI=1S/C12H14N2O2/c15-11(16)10-6-5-7-8(9(6)13-14-10)12(7)3-1-2-4-12/h7-8H,1-5H2,(H,13,14)(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in CHO cells assessed as decrease in forskolin-stimulated cAMP production by HTRF assay |
J Med Chem 55: 3644-66 (2012)
Article DOI: 10.1021/jm2010964
BindingDB Entry DOI: 10.7270/Q2000353 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a |
ShanghaiTech University
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109A (unknown origin) assessed as effect on beta-arrestin2 conformational changes |
J Med Chem 61: 9841-9878 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00435
BindingDB Entry DOI: 10.7270/Q2F76GX7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50541467

(CHEMBL4647392)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1ccc2c(Cl)c(O)ccc2c1 Show InChI InChI=1S/C20H16ClNO4/c21-19-14-8-5-12(11-13(14)7-9-17(19)23)6-10-18(24)22-16-4-2-1-3-15(16)20(25)26/h1-5,7-9,11,23H,6,10H2,(H,22,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109A receptor (unknown origin) expressed in HEK293T cell membranes after 30 mins by [35S]GTPgammaS binding based microbeta sci... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127105
BindingDB Entry DOI: 10.7270/Q2PZ5DC8 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384645
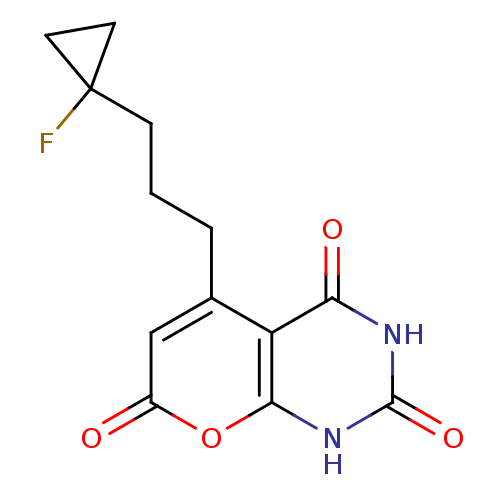
(CHEMBL2035002)Show SMILES FC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C13H13FN2O4/c14-13(4-5-13)3-1-2-7-6-8(17)20-11-9(7)10(18)15-12(19)16-11/h6H,1-5H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384647

(CHEMBL2036959)Show SMILES CCC1(CCCc2cc(=O)oc3[nH]c(=O)[nH]c(=O)c23)CC1 Show InChI InChI=1S/C15H18N2O4/c1-2-15(6-7-15)5-3-4-9-8-10(18)21-13-11(9)12(19)16-14(20)17-13/h8H,2-7H2,1H3,(H2,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313976
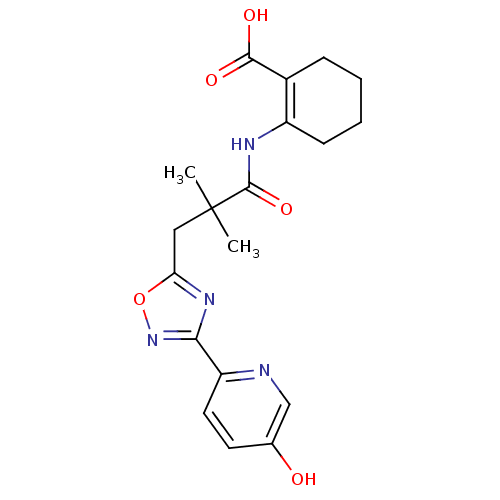
(2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...)Show SMILES CC(C)(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:21| Show InChI InChI=1S/C19H22N4O5/c1-19(2,18(27)21-13-6-4-3-5-12(13)17(25)26)9-15-22-16(23-28-15)14-8-7-11(24)10-20-14/h7-8,10,24H,3-6,9H2,1-2H3,(H,21,27)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at cloned human GPR109A receptor expressed in CHO-K1 cells by [35S]GTPgammaS binding assay |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50319109

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CCCC1CCC(NC(=O)CCc2nc(no2)-c2ccc(O)cn2)=C(C1)C(O)=O |c:25| Show InChI InChI=1S/C20H24N4O5/c1-2-3-12-4-6-15(14(10-12)20(27)28)22-17(26)8-9-18-23-19(24-29-18)16-7-5-13(25)11-21-16/h5,7,11-12,25H,2-4,6,8-10H2,1H3,(H,22,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384616
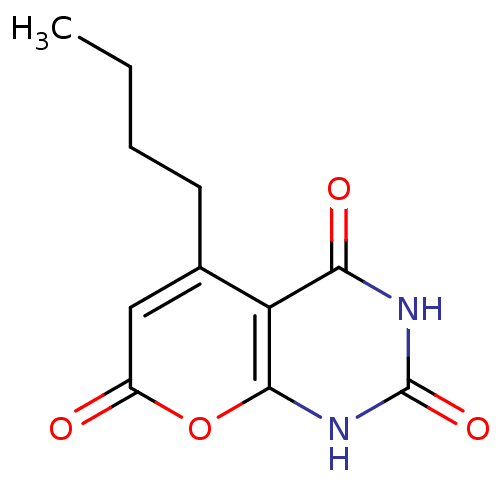
(CHEMBL2036813)Show InChI InChI=1S/C11H12N2O4/c1-2-3-4-6-5-7(14)17-10-8(6)9(15)12-11(16)13-10/h5H,2-4H2,1H3,(H2,12,13,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a |
ShanghaiTech University
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109A (unknown origin) assessed as inhibition of forskolin-mediated cAMP accumulation after 15 mins by fluorescence assay |
J Med Chem 61: 9841-9878 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00435
BindingDB Entry DOI: 10.7270/Q2F76GX7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384627
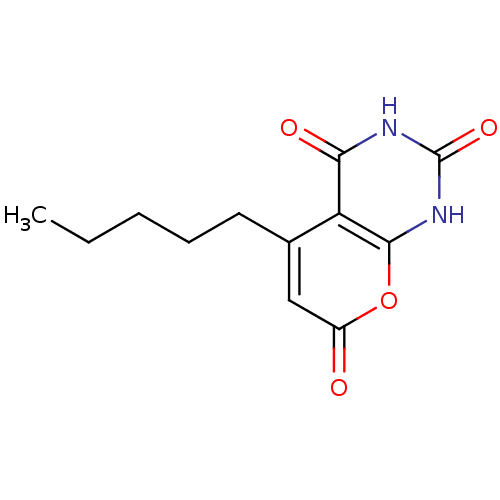
(CHEMBL2036825)Show InChI InChI=1S/C12H14N2O4/c1-2-3-4-5-7-6-8(15)18-11-9(7)10(16)13-12(17)14-11/h6H,2-5H2,1H3,(H2,13,14,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277714
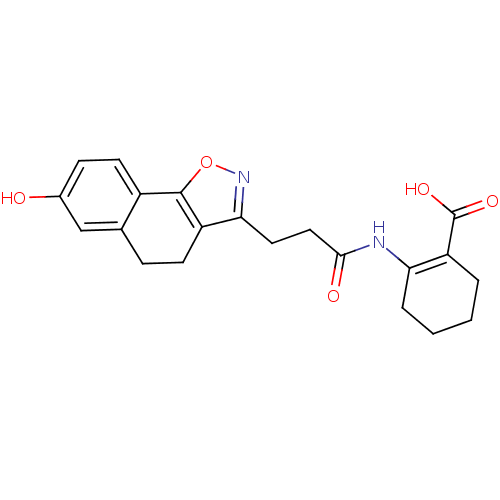
(2-(3-(7-hydroxy-4,5-dihydronaphtho[2,1-d]isoxazol-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc-2c1CCc1cc(O)ccc-21 |t:3| Show InChI InChI=1S/C21H22N2O5/c24-13-6-8-14-12(11-13)5-7-15-18(23-28-20(14)15)9-10-19(25)22-17-4-2-1-3-16(17)21(26)27/h6,8,11,24H,1-5,7,9-10H2,(H,22,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in HEK293 cells harboring glosensor-22F cAMP plasmid DNA assessed as inhibition of forskolin-stimulated c... |
Eur J Med Chem 152: 208-222 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.037
BindingDB Entry DOI: 10.7270/Q2B27XT0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109a expressed in HEK293 cells harboring glosensor-22F cAMP plasmid DNA assessed as inhibition of forskolin-stimulated c... |
Eur J Med Chem 152: 208-222 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.037
BindingDB Entry DOI: 10.7270/Q2B27XT0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977
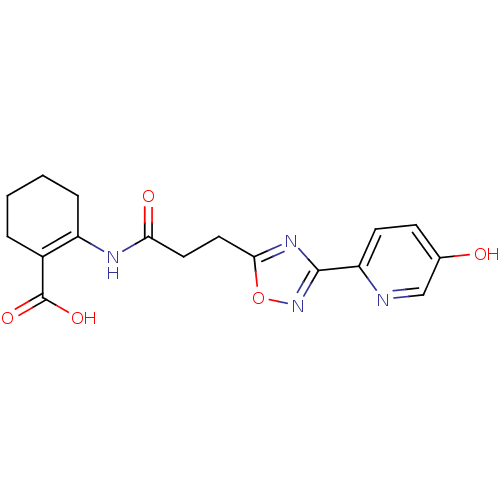
(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at cloned human GPR109A receptor expressed in CHO-K1 cells by [35S]GTPgammaS binding assay |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50362630

(CHEMBL1939047)Show InChI InChI=1S/C18H20N2O4/c1-2-3-4-10-23-18-19-16(22)15-13(9-8-12-6-5-7-12)11-14(21)24-17(15)20-18/h11-12H,2,5-10H2,1H3,(H,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Agonist activity at human HM74A |
Bioorg Med Chem Lett 22: 854-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.041
BindingDB Entry DOI: 10.7270/Q24B31S5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50319118

(5-(3,5-difluorophenyl)-2-(3-(3-(5-fluoropyridin-2-...)Show SMILES OC(=O)C1=C(CCC(C1)c1cc(F)cc(F)c1)NC(=O)CCc1nc(no1)-c1ccc(F)cn1 |t:3| Show InChI InChI=1S/C23H19F3N4O4/c24-14-2-4-19(27-11-14)22-29-21(34-30-22)6-5-20(31)28-18-3-1-12(9-17(18)23(32)33)13-7-15(25)10-16(26)8-13/h2,4,7-8,10-12H,1,3,5-6,9H2,(H,28,31)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human niacin receptor expressed in CHO-KI cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 3426-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.001
BindingDB Entry DOI: 10.7270/Q2W0963X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313977

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1nc(no1)-c1ccc(O)cn1 |t:3| Show InChI InChI=1S/C17H18N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h5-6,9,22H,1-4,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109A receptor expressed in CHO-K1 cells after 60 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2721-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.116
BindingDB Entry DOI: 10.7270/Q2N29X8C |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50277716
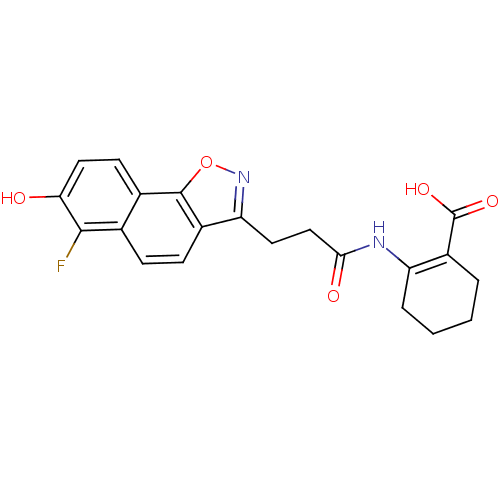
(2-(3-(6-fluoro-7-hydroxynaphtho[2,1-d]isoxazol-3-y...)Show SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1noc2c1ccc1c(F)c(O)ccc21 |t:3| Show InChI InChI=1S/C21H19FN2O5/c22-19-11-5-6-13-16(24-29-20(13)12(11)7-9-17(19)25)8-10-18(26)23-15-4-2-1-3-14(15)21(27)28/h5-7,9,25H,1-4,8,10H2,(H,23,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assay |
J Med Chem 52: 2587-602 (2009)
Article DOI: 10.1021/jm900151e
BindingDB Entry DOI: 10.7270/Q2RJ4JDG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at cloned human GPR109A receptor expressed in CHO-K1 cells by [35S]GTPgammaS binding assay |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50313978

(2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES CC(Cc1nc(no1)-c1ccc(O)cn1)C(=O)NC1=C(CCCC1)C(O)=O |t:20| Show InChI InChI=1S/C18H20N4O5/c1-10(17(24)20-13-5-3-2-4-12(13)18(25)26)8-15-21-16(22-27-15)14-7-6-11(23)9-19-14/h6-7,9-10,23H,2-5,8H2,1H3,(H,20,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at cloned human GPR109A receptor expressed in CHO-K1 cells by [35S]GTPgammaS binding assay |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50362626
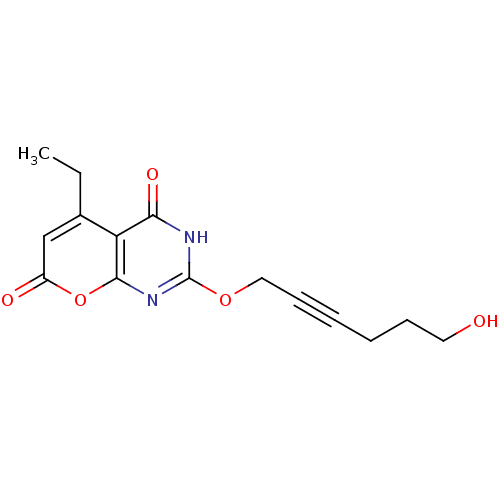
(CHEMBL1939043)Show InChI InChI=1S/C15H16N2O5/c1-2-10-9-11(19)22-14-12(10)13(20)16-15(17-14)21-8-6-4-3-5-7-18/h9,18H,2-3,5,7-8H2,1H3,(H,16,17,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Agonist activity at human HM74A |
Bioorg Med Chem Lett 22: 854-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.041
BindingDB Entry DOI: 10.7270/Q24B31S5 |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM50384626

(CHEMBL2036823)Show InChI InChI=1S/C14H12N2O4S/c17-10-7-8(3-1-4-9-5-2-6-21-9)11-12(18)15-14(19)16-13(11)20-10/h2,5-7H,1,3-4H2,(H2,15,16,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109a expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation |
ACS Med Chem Lett 3: 63-68 (2012)
Article DOI: 10.1021/ml200243g
BindingDB Entry DOI: 10.7270/Q29K4C8X |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533
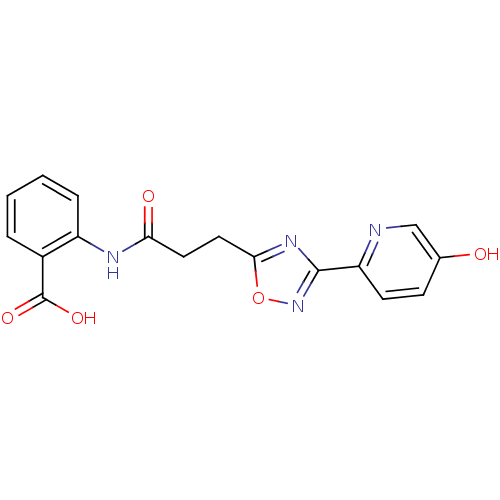
(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant GPR109A receptor expressed in CHO-K1 cells after 60 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 21: 2721-4 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.116
BindingDB Entry DOI: 10.7270/Q2N29X8C |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23515

(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at GPR109a receptor transfected in CHOK1 cells assessed as inhibition of forskolin-induced cAMP generation by HTRF assay |
Bioorg Med Chem Lett 19: 4207-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.108
BindingDB Entry DOI: 10.7270/Q2GF0TJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533

(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | 27 | n/a | n/a | 7.4 | 23 |
Merck Research Laboratories
| Assay Description
Membranes were incubated in binding buffer with [5, 6-3H]-niacin in the presence of test compound. After 4 hours at room temperature, reactions were ... |
J Med Chem 50: 6303-6 (2007)
Article DOI: 10.1021/jm700942d
BindingDB Entry DOI: 10.7270/Q29S1PBG |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Homo sapiens (Human)) | BDBM23533

(2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...)Show SMILES OC(=O)c1ccccc1NC(=O)CCc1nc(no1)-c1ccc(O)cn1 Show InChI InChI=1S/C17H14N4O5/c22-10-5-6-13(18-9-10)16-20-15(26-21-16)8-7-14(23)19-12-4-2-1-3-11(12)17(24)25/h1-6,9,22H,7-8H2,(H,19,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at cloned human GPR109A receptor expressed in CHO-K1 cells by [35S]GTPgammaS binding assay |
J Med Chem 53: 2666-70 (2010)
Article DOI: 10.1021/jm100022r
BindingDB Entry DOI: 10.7270/Q2NS0V2P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data