

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50009146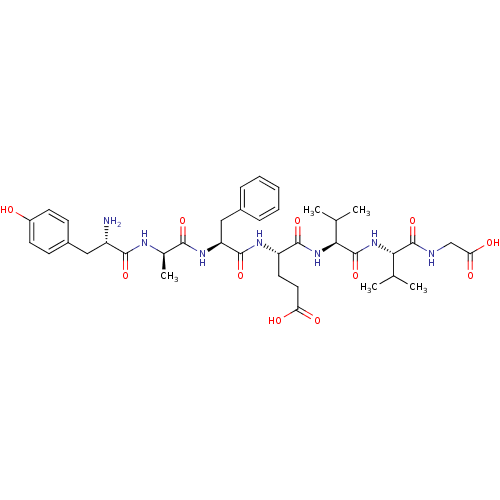 ((S)-4-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50138770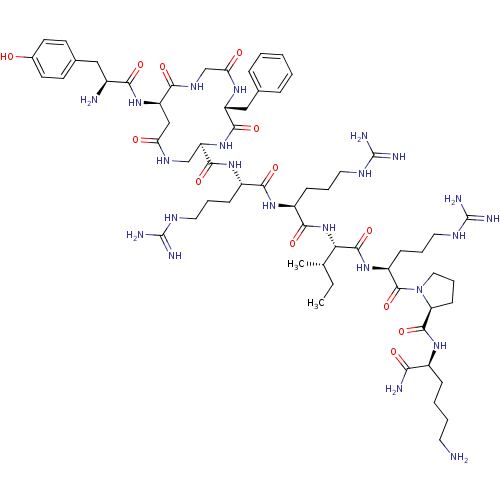 (CHEMBL411282 | cyclo[D-Asp2,Dap5]Dyn A-(1-11)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 47: 446-55 (2004) Article DOI: 10.1021/jm030298e BindingDB Entry DOI: 10.7270/Q2Q23ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... | J Med Chem 52: 5619-25 (2009) Article DOI: 10.1021/jm900577k BindingDB Entry DOI: 10.7270/Q2QN66T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50325534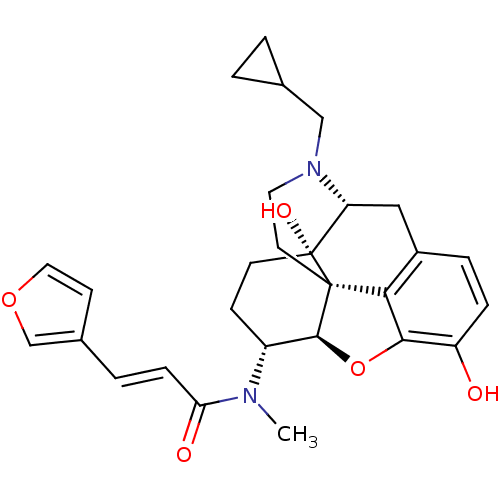 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in ERK1/2 phosphorylation after 5 mins by Wes... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50138766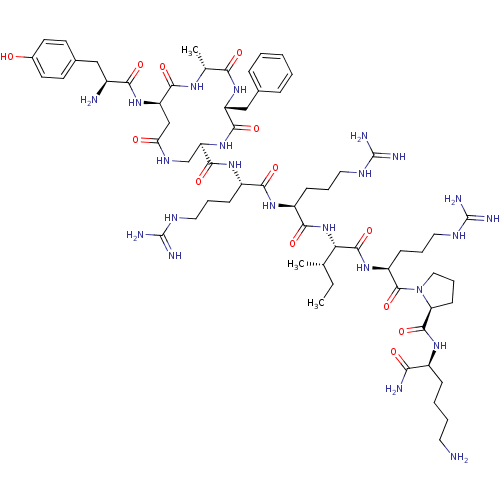 (CHEMBL439272 | cyclo[D-Ala3]Dyn A-(1-11)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 47: 446-55 (2004) Article DOI: 10.1021/jm030298e BindingDB Entry DOI: 10.7270/Q2Q23ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50138780 (CHEMBL261992 | cyclo[Ala3]Dyn A-(1-11)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 47: 446-55 (2004) Article DOI: 10.1021/jm030298e BindingDB Entry DOI: 10.7270/Q2Q23ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535347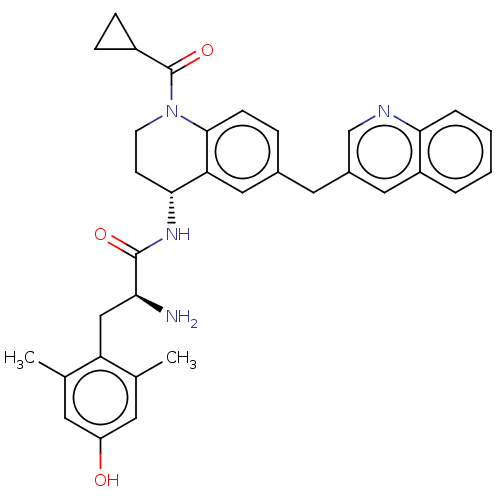 (CHEMBL4476818) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297345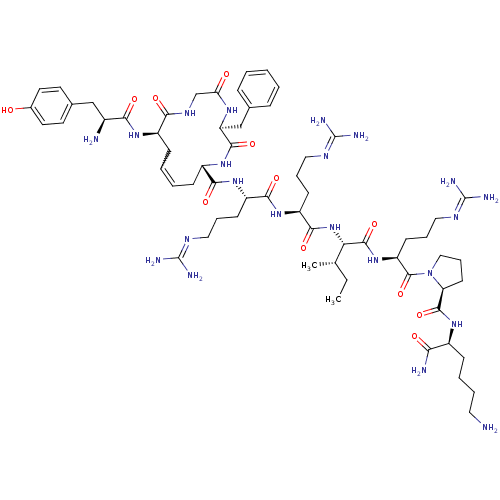 ((5S,8S,13R,Z)-13-((S)-2-amino-3-(4-hydroxyphenyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... | J Med Chem 52: 5619-25 (2009) Article DOI: 10.1021/jm900577k BindingDB Entry DOI: 10.7270/Q2QN66T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274686 (CHEMBL4129679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133053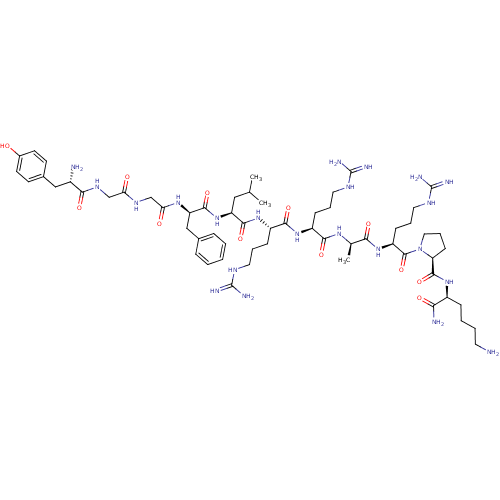 (CHEMBL405057 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21008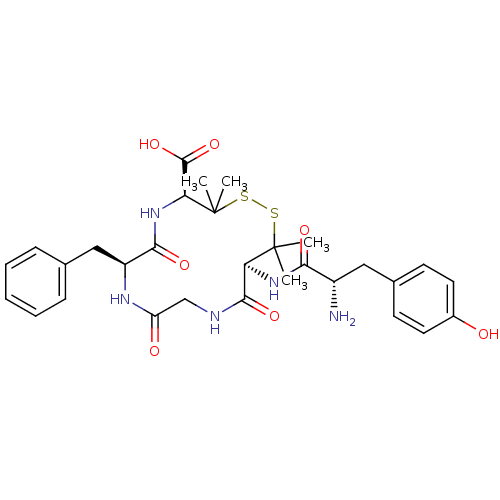 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at rat delta opioid receptor expressed in CHO cells incubated for 1 hr by [35S]GTPgammaS binding based liquid scintillation counting... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112070 BindingDB Entry DOI: 10.7270/Q2JW8JHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535339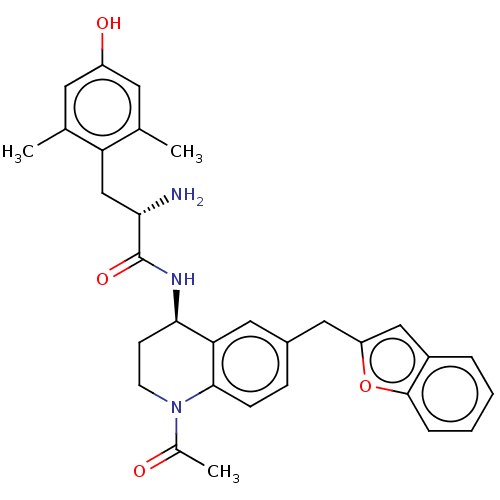 (CHEMBL4453526) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535337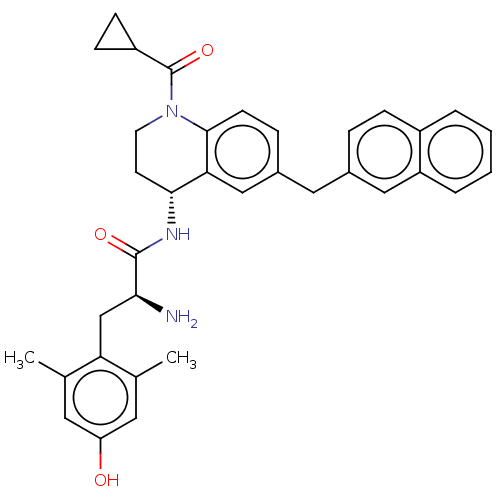 (CHEMBL4446945) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274689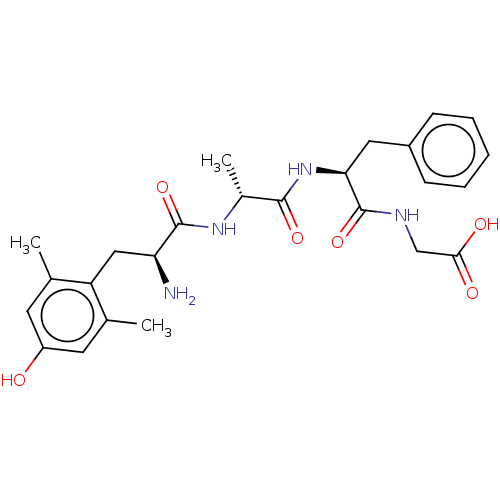 (CHEMBL4126050) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133054 (CHEMBL412228 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535349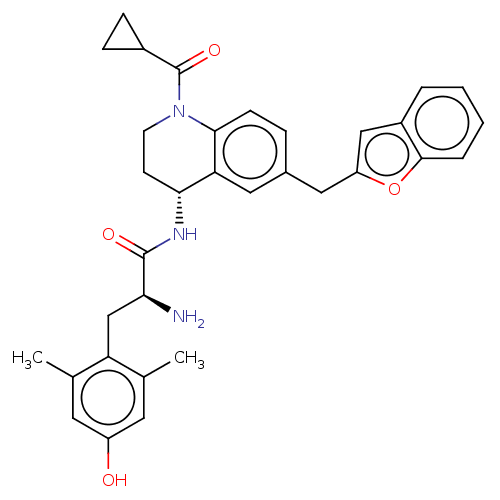 (CHEMBL4451910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50138777 (CHEMBL414904 | cyclo[Trp3]Dyn A-(1-11)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 47: 446-55 (2004) Article DOI: 10.1021/jm030298e BindingDB Entry DOI: 10.7270/Q2Q23ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274679 (CHEMBL4128530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274741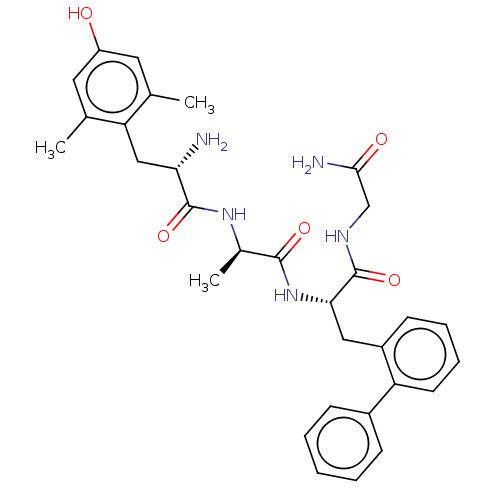 (CHEMBL4126470) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasma | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185312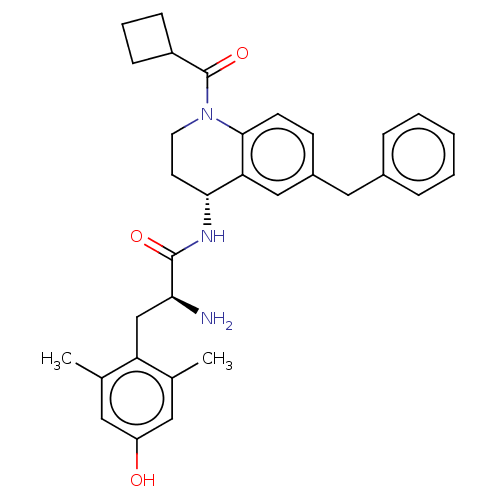 (CHEMBL3823787) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133051 (CHEMBL413832 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50325534 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged rat kappa opioid receptor expressed in HEK293 cells assessed as increase in beta-arrestin mediated p38 phosphorylation... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274694 (CHEMBL4129901) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535343 (CHEMBL4550332) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effective concentration to inhibit E203Q,D204N,D206N KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274696 (CHEMBL4126102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50381677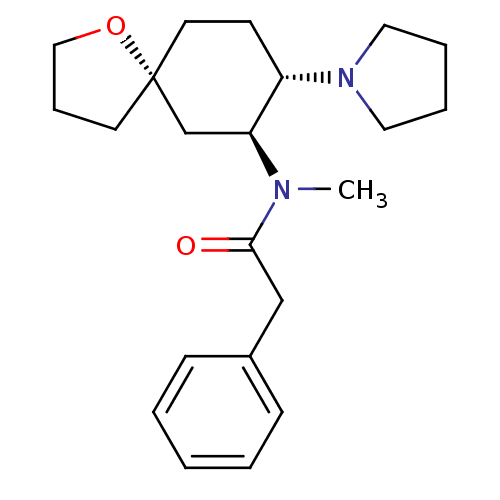 (CHEMBL1256748 | U-69593) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio" Curated by ChEMBL | Assay Description Agonist activity at rat kappa opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | ACS Med Chem Lett 5: 1032-6 (2014) Article DOI: 10.1021/ml500241n BindingDB Entry DOI: 10.7270/Q23T9JS5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effective concentration to inhibit D216,ND217N,E218Q KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50240354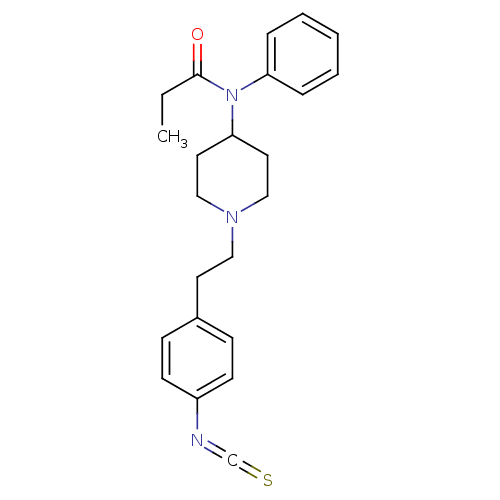 (CHEMBL274844 | N-{1-[2-(4-Isothiocyanato-phenyl)-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The binding affinity was evaluated against [3H]dalamid binding to neuroblastoma X glioma hybrid cell NG108-15 (Opioid receptor delta 1)membranes | J Med Chem 27: 1570-4 (1985) BindingDB Entry DOI: 10.7270/Q2S18328 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185309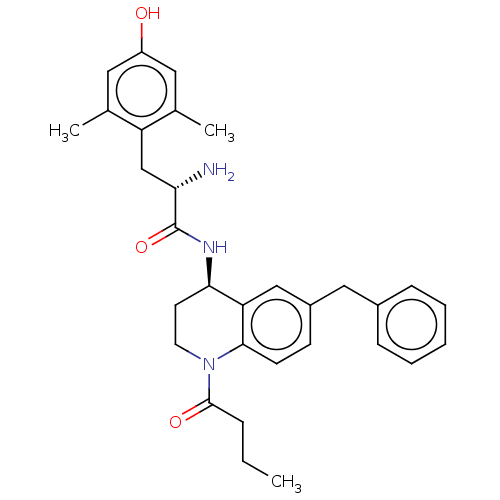 (CHEMBL3824052) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297347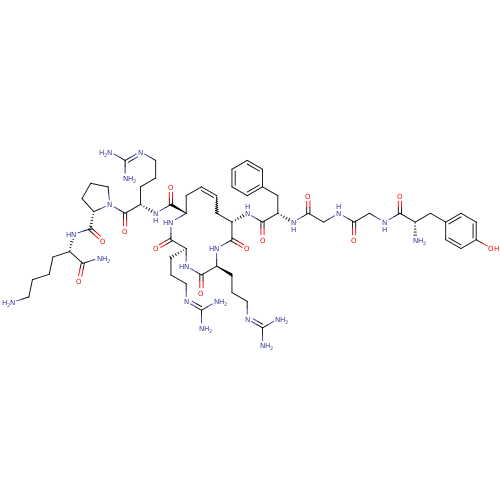 ((2S,5S,8S,13S,Z)-13-((S)-2-(2-(2-((S)-2-amino-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... | J Med Chem 52: 5619-25 (2009) Article DOI: 10.1021/jm900577k BindingDB Entry DOI: 10.7270/Q2QN66T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185311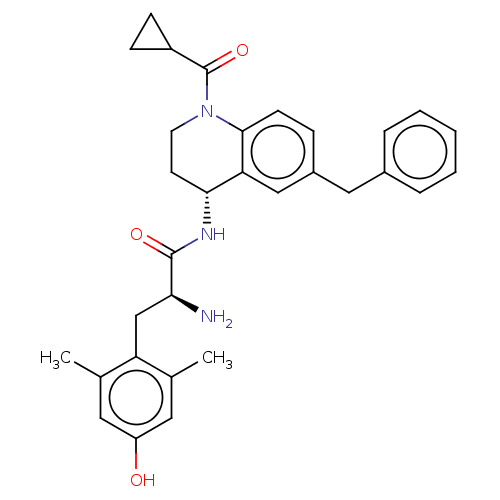 (CHEMBL3823231) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50138765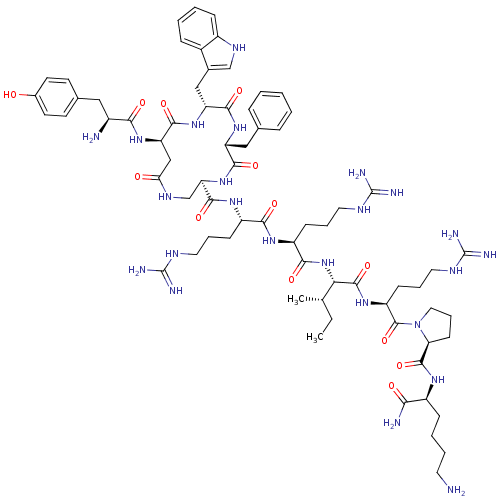 (CHEMBL405307 | cyclo[D-Trp3]Dyn A-(1-11)NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated adenylyl cyclase activity by compound in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 47: 446-55 (2004) Article DOI: 10.1021/jm030298e BindingDB Entry DOI: 10.7270/Q2Q23ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535340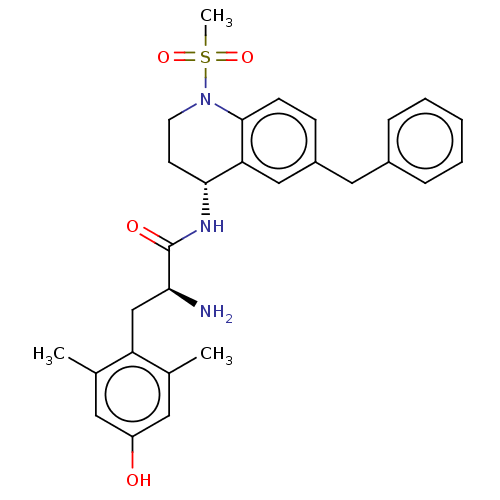 (CHEMBL4521319) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 h... | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185313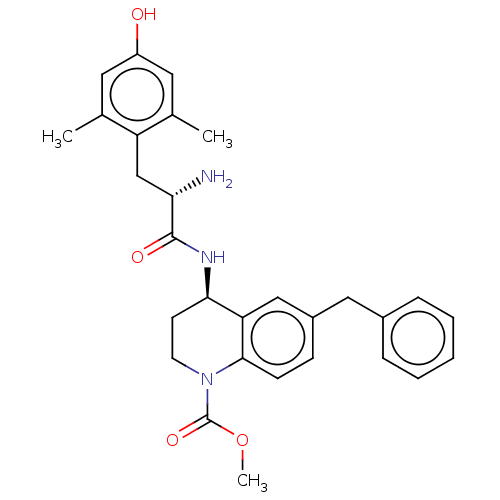 (CHEMBL3823830) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133885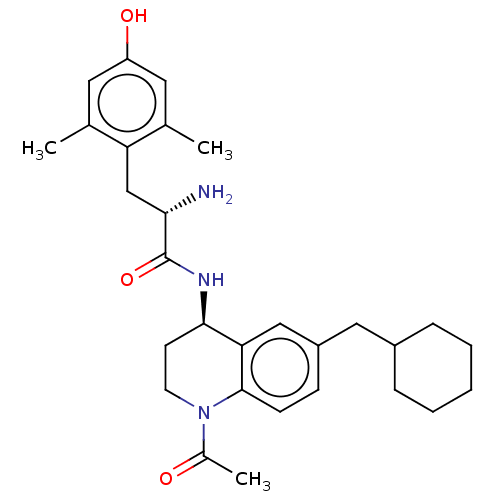 (CHEMBL3634250) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in C6 cell membrane assessed as [35S]GTPgammaS binding for 1 hr by liquid scintillation count... | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185317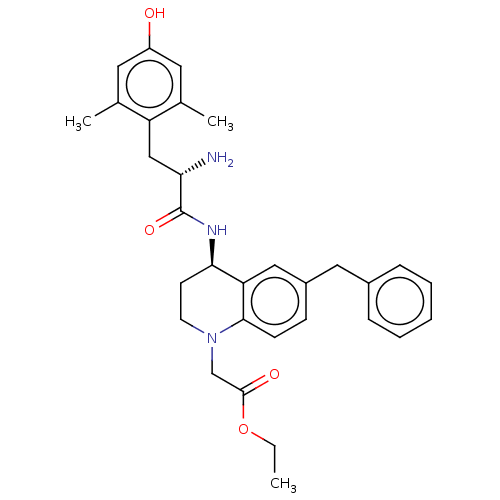 (CHEMBL3823815) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Effective concentration to inhibit wild type KL-2 Opioid receptor kappa 1 binding to [35S]GTP-gamma-S, expressed in COS cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274731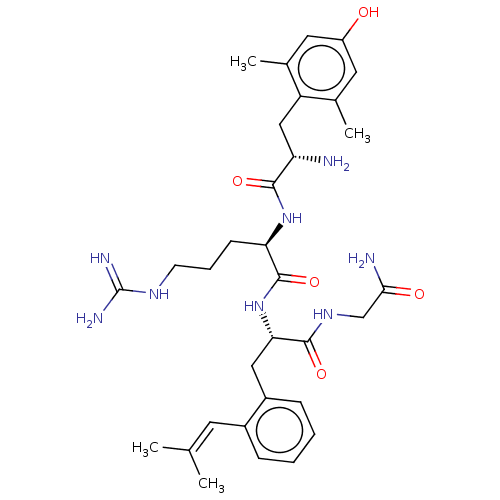 (CHEMBL4126322) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027091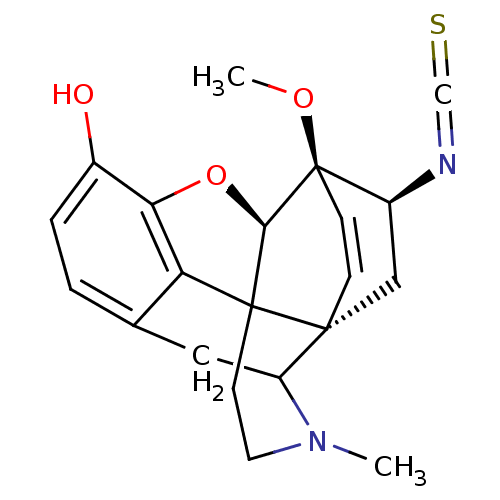 (19-isothiocyanato-15-methoxy-3-methyl-13-oxa-3-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The binding affinity was evaluated against [3H]dalamid binding to neuroblastoma X glioma hybrid cell NG108-15 (Opioid receptor delta 1)membranes | J Med Chem 27: 1570-4 (1985) BindingDB Entry DOI: 10.7270/Q2S18328 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50506099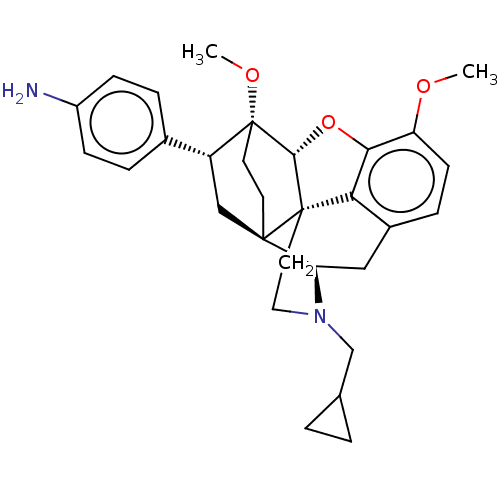 (CHEMBL4451186) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Agonist activity at rat DOR expressed in CHO cell membranes assessed as stimulation of [35S]GTPgammaS binding incubated for 1 hr by liquid scintillat... | J Med Chem 62: 11054-11070 (2019) Article DOI: 10.1021/acs.jmedchem.9b00857 BindingDB Entry DOI: 10.7270/Q2VD72RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50142699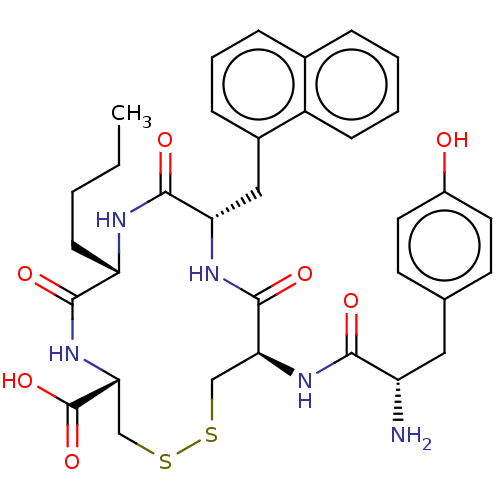 (CHEMBL3759831) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor transfected in C6 cells by [35S]GTPgammaS binding assay | Eur J Med Chem 108: 211-28 (2016) Article DOI: 10.1016/j.ejmech.2015.11.028 BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50142697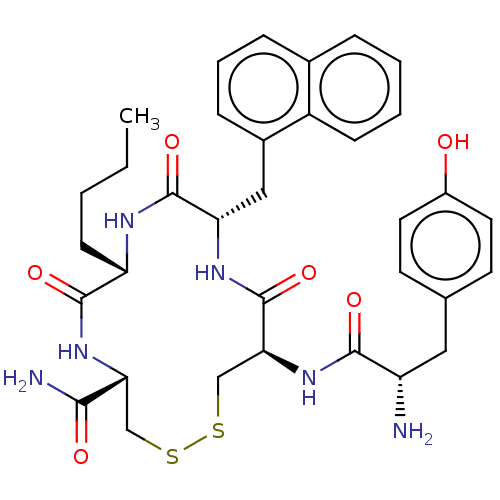 (CHEMBL3759294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor transfected in C6 cells by [35S]GTPgammaS binding assay | Eur J Med Chem 108: 211-28 (2016) Article DOI: 10.1016/j.ejmech.2015.11.028 BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297347 ((2S,5S,8S,13S,Z)-13-((S)-2-(2-(2-((S)-2-amino-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at rat cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production by scintillat... | J Med Chem 52: 5619-25 (2009) Article DOI: 10.1021/jm900577k BindingDB Entry DOI: 10.7270/Q2QN66T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185310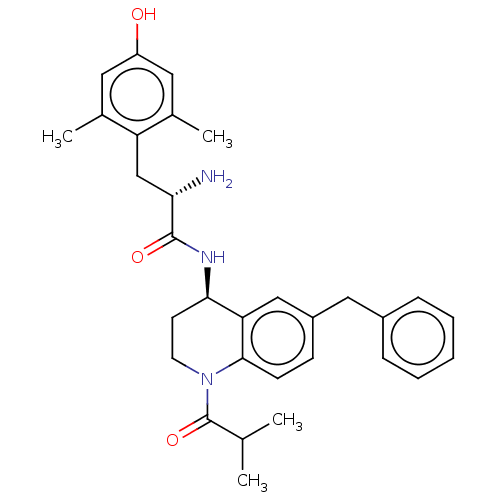 (CHEMBL3824042) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274729 (CHEMBL4128945) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat delta-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133049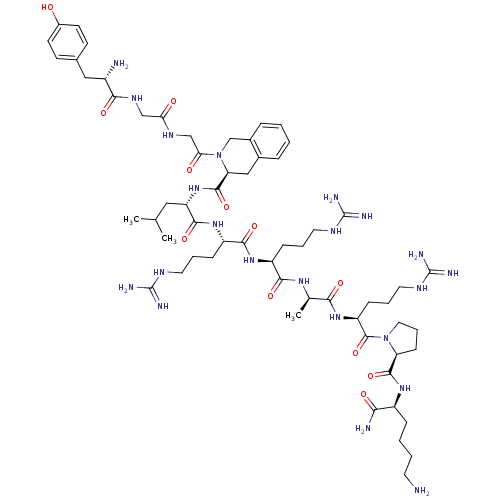 (CHEMBL262310 | Opioid Peptide [d-Ala(8)]Dynorphin ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 29.4 | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of Forskolin-Stimulated Adenylyl Cyclase in CHO cells expressing Opioid receptor kappa 1 | J Med Chem 46: 4002-8 (2003) Article DOI: 10.1021/jm030075o BindingDB Entry DOI: 10.7270/Q2CN739H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50463295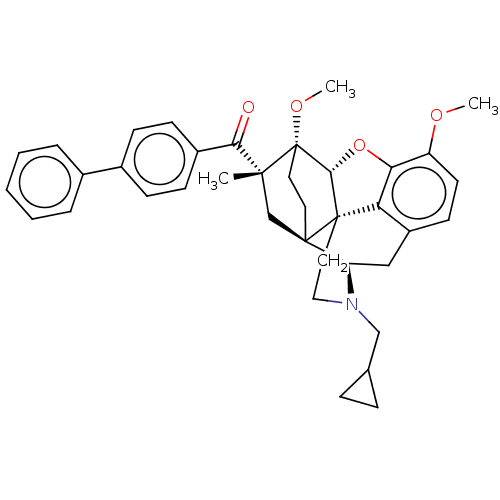 (CHEMBL4245987) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Agonist activity at rat DOR expressed in CHO cell membranes after 1 hr by [35S]GTPgammaS binding based liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185308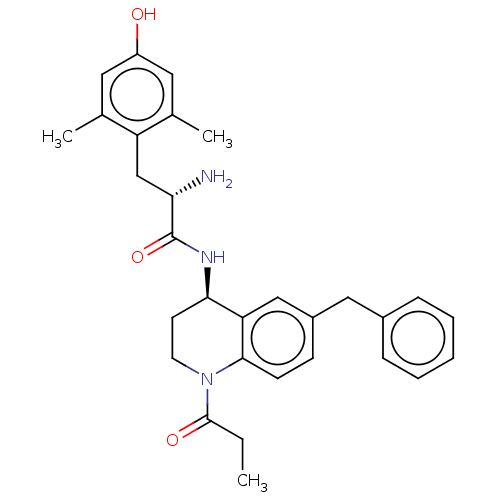 (CHEMBL3822956) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat DOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299209 ((5R,8S,11S,14S)-14-((S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat delta opioid receptor expressed in rat C6 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 mins ... | J Med Chem 52: 7724-31 (2009) Article DOI: 10.1021/jm9007483 BindingDB Entry DOI: 10.7270/Q2GQ6XTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 122 total ) | Next | Last >> |