

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Homo sapiens (Human)) | BDBM19854 (CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | 17 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19855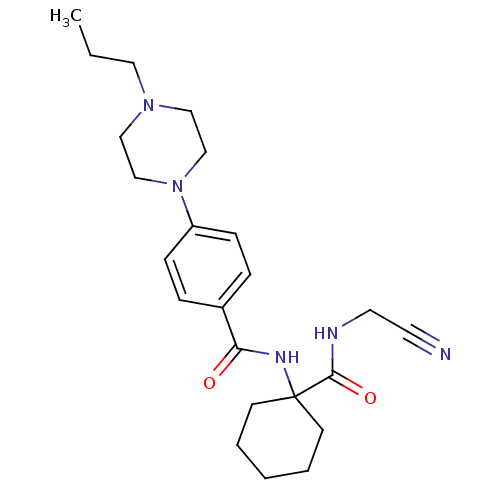 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | 61 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50391540 (CHEMBL2147469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of cathepsin B using Z-Phe-Arg-MCA as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by fluorimetric a... | J Nat Prod 75: 1546-52 (2012) Article DOI: 10.1021/np300282a BindingDB Entry DOI: 10.7270/Q2Z03979 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50391541 (CHEMBL2147467) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of cathepsin B using Z-Phe-Arg-MCA as substrate incubated for 10 mins prior to substrate addition measured after 30 mins by fluorimetric a... | J Nat Prod 75: 1546-52 (2012) Article DOI: 10.1021/np300282a BindingDB Entry DOI: 10.7270/Q2Z03979 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19859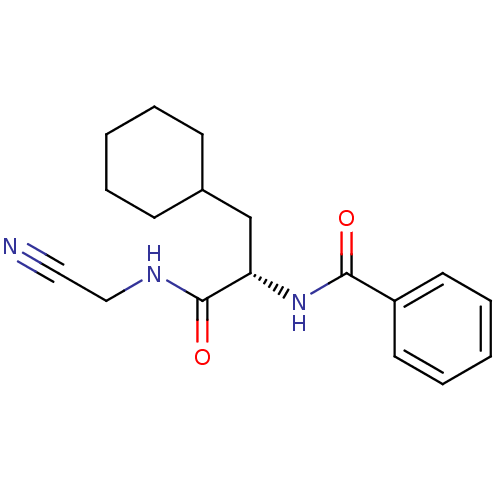 ((2S)-N-(cyanomethyl)-3-cyclohexyl-2-(phenylformami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | 940 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19857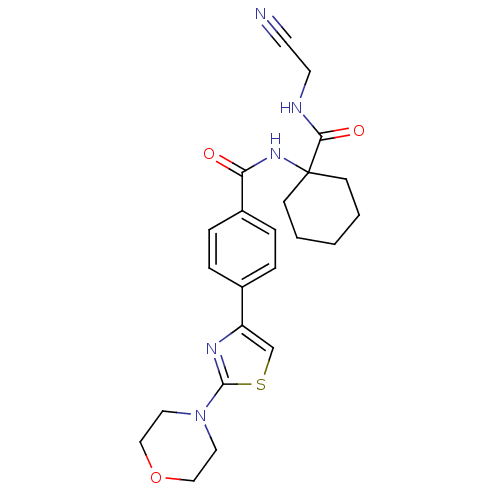 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-[2-(mor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | 2.90E+3 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19856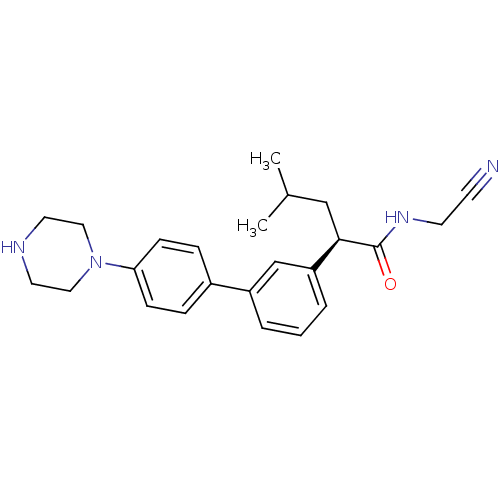 ((2R)-N-(cyanomethyl)-4-methyl-2-{3-[4-(piperazin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+4 | n/a | 4.40E+3 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM19858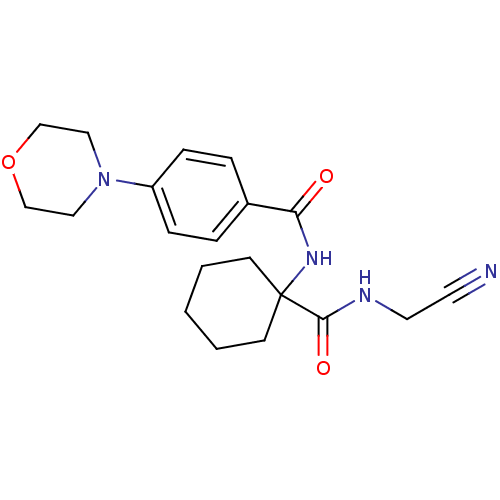 (N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | 4.40E+3 | n/a | n/a | 6.0 | 22 |
Merck Frosst Centre for Therapeutic Research | Assay Description Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ... | J Med Chem 48: 7535-43 (2005) Article DOI: 10.1021/jm0504961 BindingDB Entry DOI: 10.7270/Q28S4N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||