 Found 477 hits of ec50 data for polymerid = 49000892,49000894,50006045
Found 477 hits of ec50 data for polymerid = 49000892,49000894,50006045 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536275

(CHEMBL4520203)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C98H150N24O26S4/c1-5-55(3)83-95(145)113-63(35-37-73(99)125)87(137)115-67(49-75(101)127)89(139)117-69(53-151-149-45-39-81(133)109-65(91(141)119-83)47-57-27-31-59(123)32-28-57)97(147)121-43-19-23-71(121)93(143)111-61(85(135)107-51-77(103)129)21-15-17-41-105-79(131)25-13-11-9-7-8-10-12-14-26-80(132)106-42-18-16-22-62(86(136)108-52-78(104)130)112-94(144)72-24-20-44-122(72)98(148)70-54-152-150-46-40-82(134)110-66(48-58-29-33-60(124)34-30-58)92(142)120-84(56(4)6-2)96(146)114-64(36-38-74(100)126)88(138)116-68(50-76(102)128)90(140)118-70/h27-34,55-56,61-72,83-84,123-124H,5-26,35-54H2,1-4H3,(H2,99,125)(H2,100,126)(H2,101,127)(H2,102,128)(H2,103,129)(H2,104,130)(H,105,131)(H,106,132)(H,107,135)(H,108,136)(H,109,133)(H,110,134)(H,111,143)(H,112,144)(H,113,145)(H,114,146)(H,115,137)(H,116,138)(H,117,139)(H,118,140)(H,119,141)(H,120,142)/t55-,56-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,83-,84-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor C47A mutant high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GF... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536278

(CHEMBL4520170)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C94H142N24O26S4/c1-5-51(3)79-91(141)109-59(31-33-69(95)121)83(133)111-63(45-71(97)123)85(135)113-65(49-147-145-41-35-77(129)105-61(87(137)115-79)43-53-23-27-55(119)28-24-53)93(143)117-39-15-19-67(117)89(139)107-57(81(131)103-47-73(99)125)17-11-13-37-101-75(127)21-9-7-8-10-22-76(128)102-38-14-12-18-58(82(132)104-48-74(100)126)108-90(140)68-20-16-40-118(68)94(144)66-50-148-146-42-36-78(130)106-62(44-54-25-29-56(120)30-26-54)88(138)116-80(52(4)6-2)92(142)110-60(32-34-70(96)122)84(134)112-64(46-72(98)124)86(136)114-66/h23-30,51-52,57-68,79-80,119-120H,5-22,31-50H2,1-4H3,(H2,95,121)(H2,96,122)(H2,97,123)(H2,98,124)(H2,99,125)(H2,100,126)(H,101,127)(H,102,128)(H,103,131)(H,104,132)(H,105,129)(H,106,130)(H,107,139)(H,108,140)(H,109,141)(H,110,142)(H,111,133)(H,112,134)(H,113,135)(H,114,136)(H,115,137)(H,116,138)/t51-,52-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,79-,80-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GFP-tagged Gga... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536279
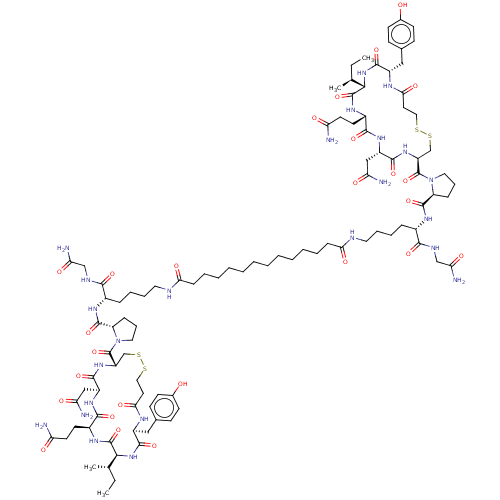
(CHEMBL4567752)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCCCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C100H154N24O26S4/c1-5-57(3)85-97(147)115-65(37-39-75(101)127)89(139)117-69(51-77(103)129)91(141)119-71(55-153-151-47-41-83(135)111-67(93(143)121-85)49-59-29-33-61(125)34-30-59)99(149)123-45-21-25-73(123)95(145)113-63(87(137)109-53-79(105)131)23-17-19-43-107-81(133)27-15-13-11-9-7-8-10-12-14-16-28-82(134)108-44-20-18-24-64(88(138)110-54-80(106)132)114-96(146)74-26-22-46-124(74)100(150)72-56-154-152-48-42-84(136)112-68(50-60-31-35-62(126)36-32-60)94(144)122-86(58(4)6-2)98(148)116-66(38-40-76(102)128)90(140)118-70(52-78(104)130)92(142)120-72/h29-36,57-58,63-74,85-86,125-126H,5-28,37-56H2,1-4H3,(H2,101,127)(H2,102,128)(H2,103,129)(H2,104,130)(H2,105,131)(H2,106,132)(H,107,133)(H,108,134)(H,109,137)(H,110,138)(H,111,135)(H,112,136)(H,113,145)(H,114,146)(H,115,147)(H,116,148)(H,117,139)(H,118,140)(H,119,141)(H,120,142)(H,121,143)(H,122,144)/t57-,58-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,85-,86-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor C47A mutant high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GF... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536277
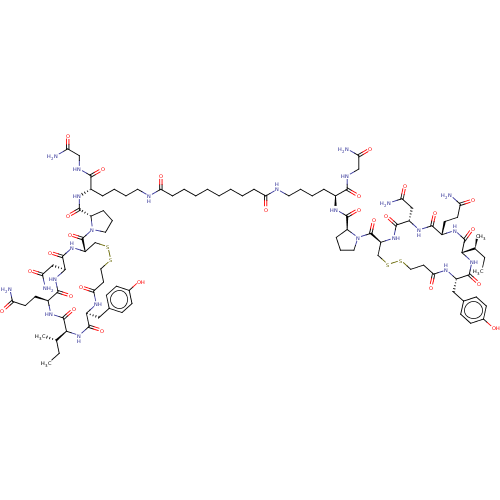
(CHEMBL4541104)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C96H146N24O26S4/c1-5-53(3)81-93(143)111-61(33-35-71(97)123)85(135)113-65(47-73(99)125)87(137)115-67(51-149-147-43-37-79(131)107-63(89(139)117-81)45-55-25-29-57(121)30-26-55)95(145)119-41-17-21-69(119)91(141)109-59(83(133)105-49-75(101)127)19-13-15-39-103-77(129)23-11-9-7-8-10-12-24-78(130)104-40-16-14-20-60(84(134)106-50-76(102)128)110-92(142)70-22-18-42-120(70)96(146)68-52-150-148-44-38-80(132)108-64(46-56-27-31-58(122)32-28-56)90(140)118-82(54(4)6-2)94(144)112-62(34-36-72(98)124)86(136)114-66(48-74(100)126)88(138)116-68/h25-32,53-54,59-70,81-82,121-122H,5-24,33-52H2,1-4H3,(H2,97,123)(H2,98,124)(H2,99,125)(H2,100,126)(H2,101,127)(H2,102,128)(H,103,129)(H,104,130)(H,105,133)(H,106,134)(H,107,131)(H,108,132)(H,109,141)(H,110,142)(H,111,143)(H,112,144)(H,113,135)(H,114,136)(H,115,137)(H,116,138)(H,117,139)(H,118,140)/t53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,81-,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GFP-tagged Gga... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536277

(CHEMBL4541104)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C96H146N24O26S4/c1-5-53(3)81-93(143)111-61(33-35-71(97)123)85(135)113-65(47-73(99)125)87(137)115-67(51-149-147-43-37-79(131)107-63(89(139)117-81)45-55-25-29-57(121)30-26-55)95(145)119-41-17-21-69(119)91(141)109-59(83(133)105-49-75(101)127)19-13-15-39-103-77(129)23-11-9-7-8-10-12-24-78(130)104-40-16-14-20-60(84(134)106-50-76(102)128)110-92(142)70-22-18-42-120(70)96(146)68-52-150-148-44-38-80(132)108-64(46-56-27-31-58(122)32-28-56)90(140)118-82(54(4)6-2)94(144)112-62(34-36-72(98)124)86(136)114-66(48-74(100)126)88(138)116-68/h25-32,53-54,59-70,81-82,121-122H,5-24,33-52H2,1-4H3,(H2,97,123)(H2,98,124)(H2,99,125)(H2,100,126)(H2,101,127)(H2,102,128)(H,103,129)(H,104,130)(H,105,133)(H,106,134)(H,107,131)(H,108,132)(H,109,141)(H,110,142)(H,111,143)(H,112,144)(H,113,135)(H,114,136)(H,115,137)(H,116,138)(H,117,139)(H,118,140)/t53-,54-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,81-,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GFP-tagged Gga... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00460 | n/a | n/a | n/a | n/a |
Kanazawa University
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR receptor expressed in HEK293 cells assessed as intracellular calcium level measured at 3 secs interval for 5 mins by fu... |
Bioorg Med Chem 27: 3358-3363 (2019)
Article DOI: 10.1016/j.bmc.2019.06.018
BindingDB Entry DOI: 10.7270/Q2N87F60 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240674

(CHEMBL4097965)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H65N11O13S2/c1-5-22(4)36-42(66)49-26(10-11-32(44)57)38(62)50-29(17-33(45)58)39(63)52-30(20-69-68-13-12-35(60)48-28(40(64)53-36)15-23-6-8-24(55)9-7-23)43(67)54-19-25(56)16-31(54)41(65)51-27(14-21(2)3)37(61)47-18-34(46)59/h6-9,21-22,25-31,36,55-56H,5,10-20H2,1-4H3,(H2,44,57)(H2,45,58)(H2,46,59)(H,47,61)(H,48,60)(H,49,66)(H,50,62)(H,51,65)(H,52,63)(H,53,64)/t22-,25+,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240613
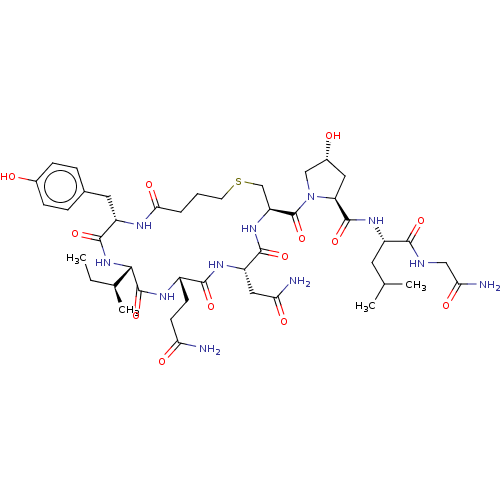
(CHEMBL4074974)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C44H67N11O13S/c1-5-23(4)37-43(67)50-27(12-13-33(45)58)39(63)51-30(18-34(46)59)40(64)53-31(21-69-14-6-7-36(61)49-29(41(65)54-37)16-24-8-10-25(56)11-9-24)44(68)55-20-26(57)17-32(55)42(66)52-28(15-22(2)3)38(62)48-19-35(47)60/h8-11,22-23,26-32,37,56-57H,5-7,12-21H2,1-4H3,(H2,45,58)(H2,46,59)(H2,47,60)(H,48,62)(H,49,61)(H,50,67)(H,51,63)(H,52,66)(H,53,64)(H,54,65)/t23-,26+,27-,28-,29-,30-,31-,32-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240638
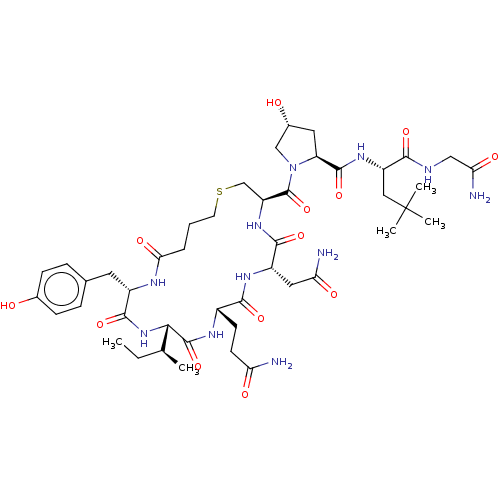
(CHEMBL4066368)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CC(C)(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C45H69N11O13S/c1-6-23(2)37-43(68)51-27(13-14-33(46)59)39(64)52-29(18-34(47)60)40(65)54-31(22-70-15-7-8-36(62)50-28(41(66)55-37)16-24-9-11-25(57)12-10-24)44(69)56-21-26(58)17-32(56)42(67)53-30(19-45(3,4)5)38(63)49-20-35(48)61/h9-12,23,26-32,37,57-58H,6-8,13-22H2,1-5H3,(H2,46,59)(H2,47,60)(H2,48,61)(H,49,63)(H,50,62)(H,51,68)(H,52,64)(H,53,67)(H,54,65)(H,55,66)/t23-,26+,27-,28-,29-,30-,31-,32-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044752
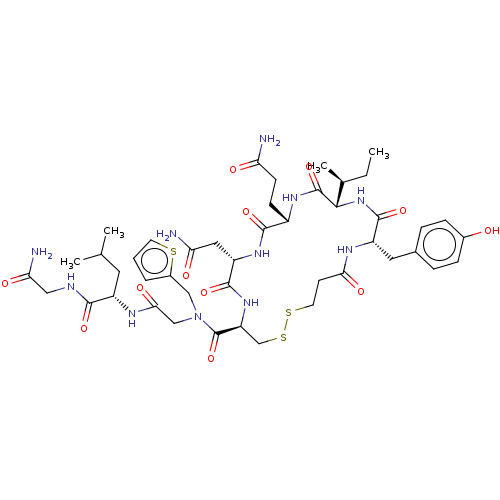
(CHEMBL3353956)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1cccs1)[C@@H](C)CC |r| Show InChI InChI=1S/C45H65N11O12S3/c1-5-25(4)39-44(67)52-29(12-13-34(46)58)41(64)53-32(19-35(47)59)42(65)54-33(23-71-70-16-14-37(61)50-31(43(66)55-39)18-26-8-10-27(57)11-9-26)45(68)56(21-28-7-6-15-69-28)22-38(62)51-30(17-24(2)3)40(63)49-20-36(48)60/h6-11,15,24-25,29-33,39,57H,5,12-14,16-23H2,1-4H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,63)(H,50,61)(H,51,62)(H,52,67)(H,53,64)(H,54,65)(H,55,66)/t25-,29-,30-,31-,32-,33-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240632
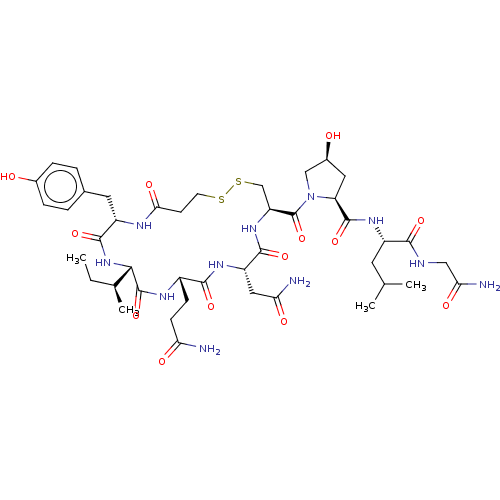
(CHEMBL4070222)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@@H](O)C[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H65N11O13S2/c1-5-22(4)36-42(66)49-26(10-11-32(44)57)38(62)50-29(17-33(45)58)39(63)52-30(20-69-68-13-12-35(60)48-28(40(64)53-36)15-23-6-8-24(55)9-7-23)43(67)54-19-25(56)16-31(54)41(65)51-27(14-21(2)3)37(61)47-18-34(46)59/h6-9,21-22,25-31,36,55-56H,5,10-20H2,1-4H3,(H2,44,57)(H2,45,58)(H2,46,59)(H,47,61)(H,48,60)(H,49,66)(H,50,62)(H,51,65)(H,52,63)(H,53,64)/t22-,25-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240633

(CHEMBL4093631)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(C)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C41H63N11O12S2/c1-6-22(4)35-40(63)48-25(11-12-30(42)54)37(60)49-28(17-31(43)55)38(61)50-29(41(64)52(5)19-34(58)47-26(15-21(2)3)36(59)45-18-32(44)56)20-66-65-14-13-33(57)46-27(39(62)51-35)16-23-7-9-24(53)10-8-23/h7-10,21-22,25-29,35,53H,6,11-20H2,1-5H3,(H2,42,54)(H2,43,55)(H2,44,56)(H,45,59)(H,46,57)(H,47,58)(H,48,63)(H,49,60)(H,50,61)(H,51,62)/t22-,25-,26-,27-,28-,29-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240651

(CHEMBL3276119)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C44H67N11O12S/c1-5-24(4)37-43(66)50-27(14-15-33(45)57)39(62)51-30(20-34(46)58)40(63)53-31(22-68-17-7-9-36(60)49-29(41(64)54-37)19-25-10-12-26(56)13-11-25)44(67)55-16-6-8-32(55)42(65)52-28(18-23(2)3)38(61)48-21-35(47)59/h10-13,23-24,27-32,37,56H,5-9,14-22H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,60)(H,50,66)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t24-,27-,28-,29-,30-,31-,32-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240610

(CHEMBL4101658)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C45H69N11O12S/c1-6-24(2)37-43(67)51-27(15-16-33(46)58)39(63)52-29(20-34(47)59)40(64)54-31(23-69-18-8-10-36(61)50-28(41(65)55-37)19-25-11-13-26(57)14-12-25)44(68)56-17-7-9-32(56)42(66)53-30(21-45(3,4)5)38(62)49-22-35(48)60/h11-14,24,27-32,37,57H,6-10,15-23H2,1-5H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,62)(H,50,61)(H,51,67)(H,52,63)(H,53,66)(H,54,64)(H,55,65)/t24-,27-,28-,29-,30-,31-,32-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240636
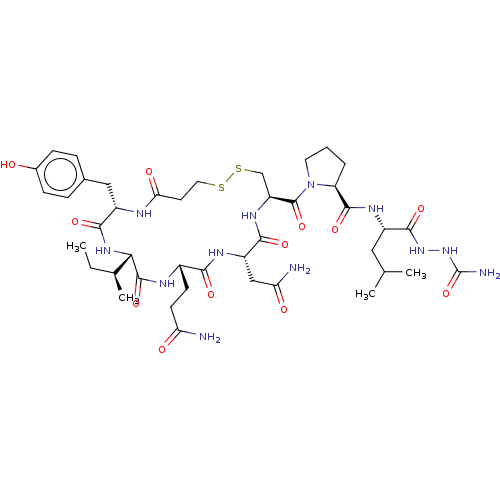
(CHEMBL4104080)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NNC(N)=O |r| Show InChI InChI=1S/C42H64N12O12S2/c1-5-22(4)34-40(64)47-25(12-13-31(43)56)35(59)48-28(19-32(44)57)36(60)50-29(20-68-67-16-14-33(58)46-27(37(61)51-34)18-23-8-10-24(55)11-9-23)41(65)54-15-6-7-30(54)39(63)49-26(17-21(2)3)38(62)52-53-42(45)66/h8-11,21-22,25-30,34,55H,5-7,12-20H2,1-4H3,(H2,43,56)(H2,44,57)(H,46,58)(H,47,64)(H,48,59)(H,49,63)(H,50,60)(H,51,61)(H,52,62)(H3,45,53,66)/t22-,25-,26-,27-,28-,29-,30-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044676
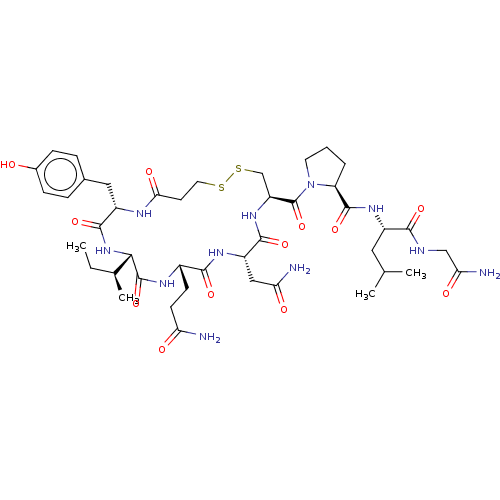
(CHEMBL439044)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C43H65N11O12S2/c1-5-23(4)36-42(65)49-26(12-13-32(44)56)38(61)50-29(19-33(45)57)39(62)52-30(21-68-67-16-14-35(59)48-28(40(63)53-36)18-24-8-10-25(55)11-9-24)43(66)54-15-6-7-31(54)41(64)51-27(17-22(2)3)37(60)47-20-34(46)58/h8-11,22-23,26-31,36,55H,5-7,12-21H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,60)(H,48,59)(H,49,65)(H,50,61)(H,51,64)(H,52,62)(H,53,63)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240634

(CHEMBL4074335)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C44H67N11O12S2/c1-6-23(2)36-42(66)50-26(13-14-32(45)57)38(62)51-28(19-33(46)58)39(63)53-30(22-69-68-17-15-35(60)49-27(40(64)54-36)18-24-9-11-25(56)12-10-24)43(67)55-16-7-8-31(55)41(65)52-29(20-44(3,4)5)37(61)48-21-34(47)59/h9-12,23,26-31,36,56H,6-8,13-22H2,1-5H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,60)(H,50,66)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044677
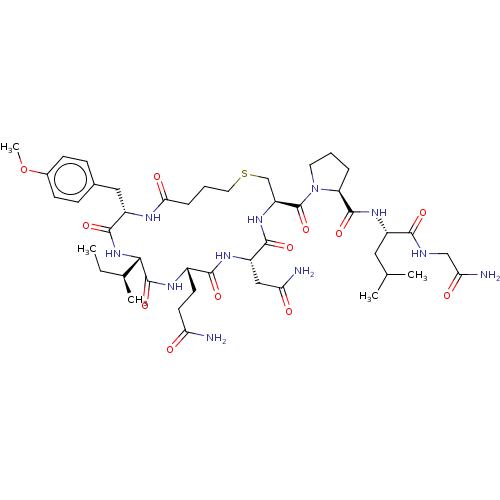
(CHEBI:59204 | Carbetocin)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(OC)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C45H69N11O12S/c1-6-25(4)38-44(66)51-28(15-16-34(46)57)40(62)52-31(21-35(47)58)41(63)54-32(23-69-18-8-10-37(60)50-30(42(64)55-38)20-26-11-13-27(68-5)14-12-26)45(67)56-17-7-9-33(56)43(65)53-29(19-24(2)3)39(61)49-22-36(48)59/h11-14,24-25,28-33,38H,6-10,15-23H2,1-5H3,(H2,46,57)(H2,47,58)(H2,48,59)(H,49,61)(H,50,60)(H,51,66)(H,52,62)(H,53,65)(H,54,63)(H,55,64)/t25-,28-,29-,30-,31-,32-,33-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human OTR expressed in HEK293 cells assessed as increase in intracellular calcium level measured at 3 secs interval f... |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044773
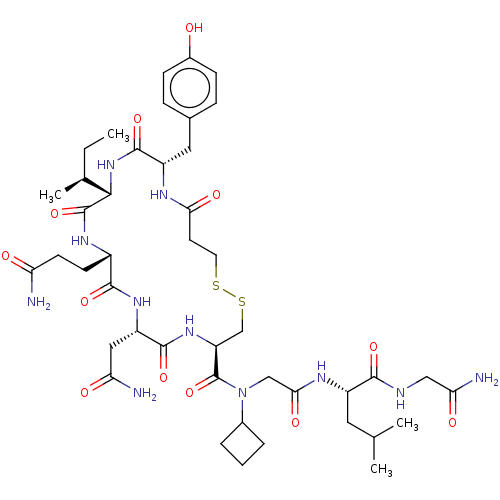
(CHEMBL3353932)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)C1CCC1)[C@@H](C)CC |r| Show InChI InChI=1S/C44H67N11O12S2/c1-5-24(4)38-43(66)51-28(13-14-33(45)57)40(63)52-31(19-34(46)58)41(64)53-32(22-69-68-16-15-36(60)49-30(42(65)54-38)18-25-9-11-27(56)12-10-25)44(67)55(26-7-6-8-26)21-37(61)50-29(17-23(2)3)39(62)48-20-35(47)59/h9-12,23-24,26,28-32,38,56H,5-8,13-22H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,62)(H,49,60)(H,50,61)(H,51,66)(H,52,63)(H,53,64)(H,54,65)/t24-,28-,29-,30-,31-,32-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044771

(CHEMBL3353939)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)C1CCCC1)[C@@H](C)CC |r| Show InChI InChI=1S/C45H69N11O12S2/c1-5-25(4)39-44(67)52-29(14-15-34(46)58)41(64)53-32(20-35(47)59)42(65)54-33(23-70-69-17-16-37(61)50-31(43(66)55-39)19-26-10-12-28(57)13-11-26)45(68)56(27-8-6-7-9-27)22-38(62)51-30(18-24(2)3)40(63)49-21-36(48)60/h10-13,24-25,27,29-33,39,57H,5-9,14-23H2,1-4H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,63)(H,50,61)(H,51,62)(H,52,67)(H,53,64)(H,54,65)(H,55,66)/t25-,29-,30-,31-,32-,33-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044770
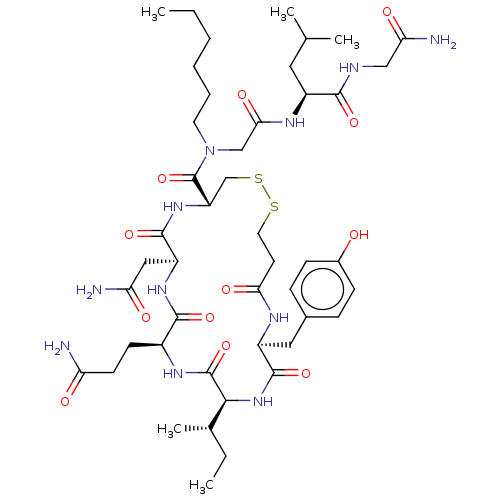
(CHEMBL3353940)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCCCC)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C46H73N11O12S2/c1-6-8-9-10-18-57(24-39(63)52-31(20-26(3)4)41(64)50-23-37(49)61)46(69)34-25-71-70-19-17-38(62)51-32(21-28-11-13-29(58)14-12-28)44(67)56-40(27(5)7-2)45(68)53-30(15-16-35(47)59)42(65)54-33(22-36(48)60)43(66)55-34/h11-14,26-27,30-34,40,58H,6-10,15-25H2,1-5H3,(H2,47,59)(H2,48,60)(H2,49,61)(H,50,64)(H,51,62)(H,52,63)(H,53,68)(H,54,65)(H,55,66)(H,56,67)/t27-,30-,31-,32-,33-,34-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044763
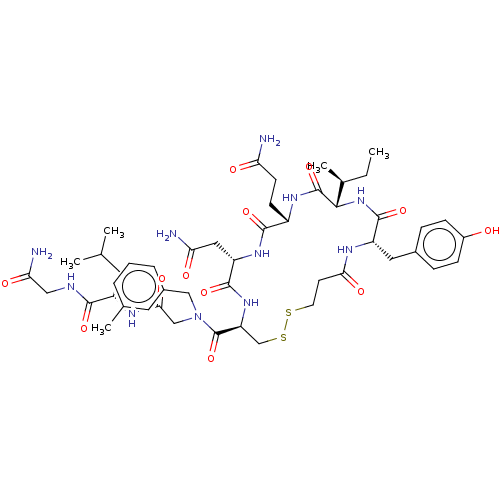
(CHEMBL3353946)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1cccc(C)c1)[C@@H](C)CC |r| Show InChI InChI=1S/C48H69N11O12S2/c1-6-28(5)42-47(70)55-32(14-15-37(49)61)44(67)56-35(21-38(50)62)45(68)57-36(25-73-72-17-16-40(64)53-34(46(69)58-42)20-29-10-12-31(60)13-11-29)48(71)59(23-30-9-7-8-27(4)19-30)24-41(65)54-33(18-26(2)3)43(66)52-22-39(51)63/h7-13,19,26,28,32-36,42,60H,6,14-18,20-25H2,1-5H3,(H2,49,61)(H2,50,62)(H2,51,63)(H,52,66)(H,53,64)(H,54,65)(H,55,70)(H,56,67)(H,57,68)(H,58,69)/t28-,32-,33-,34-,35-,36-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044761

(CHEMBL3353948)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C47H66FN11O12S2/c1-5-26(4)41-46(70)55-31(14-15-36(49)61)43(67)56-34(20-37(50)62)44(68)57-35(24-73-72-17-16-39(64)53-33(45(69)58-41)19-27-8-12-30(60)13-9-27)47(71)59(22-28-6-10-29(48)11-7-28)23-40(65)54-32(18-25(2)3)42(66)52-21-38(51)63/h6-13,25-26,31-35,41,60H,5,14-24H2,1-4H3,(H2,49,61)(H2,50,62)(H2,51,63)(H,52,66)(H,53,64)(H,54,65)(H,55,70)(H,56,67)(H,57,68)(H,58,69)/t26-,31-,32-,33-,34-,35-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044752

(CHEMBL3353956)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1cccs1)[C@@H](C)CC |r| Show InChI InChI=1S/C45H65N11O12S3/c1-5-25(4)39-44(67)52-29(12-13-34(46)58)41(64)53-32(19-35(47)59)42(65)54-33(23-71-70-16-14-37(61)50-31(43(66)55-39)18-26-8-10-27(57)11-9-26)45(68)56(21-28-7-6-15-69-28)22-38(62)51-30(17-24(2)3)40(63)49-20-36(48)60/h6-11,15,24-25,29-33,39,57H,5,12-14,16-23H2,1-4H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,63)(H,50,61)(H,51,62)(H,52,67)(H,53,64)(H,54,65)(H,55,66)/t25-,29-,30-,31-,32-,33-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240630
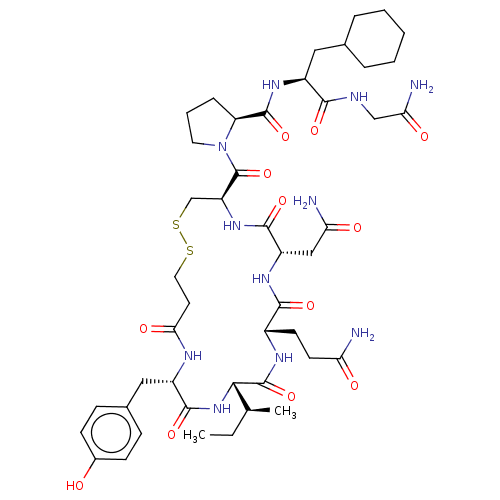
(CHEMBL4102192)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1CCCCC1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C46H69N11O12S2/c1-3-25(2)39-45(68)52-29(15-16-35(47)59)41(64)53-32(22-36(48)60)42(65)55-33(24-71-70-19-17-38(62)51-31(43(66)56-39)21-27-11-13-28(58)14-12-27)46(69)57-18-7-10-34(57)44(67)54-30(40(63)50-23-37(49)61)20-26-8-5-4-6-9-26/h11-14,25-26,29-34,39,58H,3-10,15-24H2,1-2H3,(H2,47,59)(H2,48,60)(H2,49,61)(H,50,63)(H,51,62)(H,52,68)(H,53,64)(H,54,67)(H,55,65)(H,56,66)/t25-,29-,30-,31-,32-,33-,34-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044775

(CHEMBL3353930)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC1CC1)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C44H67N11O12S2/c1-5-24(4)38-43(66)51-28(12-13-33(45)57)40(63)52-31(18-34(46)58)41(64)53-32(22-69-68-15-14-36(60)49-30(42(65)54-38)17-25-8-10-27(56)11-9-25)44(67)55(20-26-6-7-26)21-37(61)50-29(16-23(2)3)39(62)48-19-35(47)59/h8-11,23-24,26,28-32,38,56H,5-7,12-22H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,62)(H,49,60)(H,50,61)(H,51,66)(H,52,63)(H,53,64)(H,54,65)/t24-,28-,29-,30-,31-,32-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human OTR expressed in HEK293 cells assessed as increase in intracellular calcium level measured at 3 secs interval f... |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044742
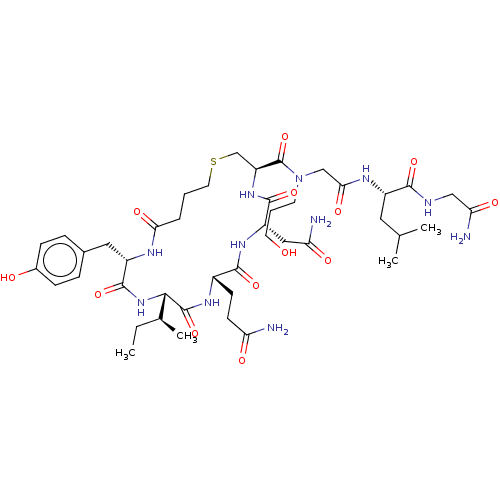
(CHEMBL3354579)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCO)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C44H69N11O13S/c1-5-25(4)38-43(67)51-28(13-14-33(45)58)40(64)52-31(20-34(46)59)41(65)53-32(23-69-17-6-8-36(61)49-30(42(66)54-38)19-26-9-11-27(57)12-10-26)44(68)55(15-7-16-56)22-37(62)50-29(18-24(2)3)39(63)48-21-35(47)60/h9-12,24-25,28-32,38,56-57H,5-8,13-23H2,1-4H3,(H2,45,58)(H2,46,59)(H2,47,60)(H,48,63)(H,49,61)(H,50,62)(H,51,67)(H,52,64)(H,53,65)(H,54,66)/t25-,28-,29-,30-,31-,32-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536279

(CHEMBL4567752)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCCCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C100H154N24O26S4/c1-5-57(3)85-97(147)115-65(37-39-75(101)127)89(139)117-69(51-77(103)129)91(141)119-71(55-153-151-47-41-83(135)111-67(93(143)121-85)49-59-29-33-61(125)34-30-59)99(149)123-45-21-25-73(123)95(145)113-63(87(137)109-53-79(105)131)23-17-19-43-107-81(133)27-15-13-11-9-7-8-10-12-14-16-28-82(134)108-44-20-18-24-64(88(138)110-54-80(106)132)114-96(146)74-26-22-46-124(74)100(150)72-56-154-152-48-42-84(136)112-68(50-60-31-35-62(126)36-32-60)94(144)122-86(58(4)6-2)98(148)116-66(38-40-76(102)128)90(140)118-70(52-78(104)130)92(142)120-72/h29-36,57-58,63-74,85-86,125-126H,5-28,37-56H2,1-4H3,(H2,101,127)(H2,102,128)(H2,103,129)(H2,104,130)(H2,105,131)(H2,106,132)(H,107,133)(H,108,134)(H,109,137)(H,110,138)(H,111,135)(H,112,136)(H,113,145)(H,114,146)(H,115,147)(H,116,148)(H,117,139)(H,118,140)(H,119,141)(H,120,142)(H,121,143)(H,122,144)/t57-,58-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,85-,86-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor V43A/C47A double mutant high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50536278

(CHEMBL4520170)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCNC(=O)CCCCCCC(=O)NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C94H142N24O26S4/c1-5-51(3)79-91(141)109-59(31-33-69(95)121)83(133)111-63(45-71(97)123)85(135)113-65(49-147-145-41-35-77(129)105-61(87(137)115-79)43-53-23-27-55(119)28-24-53)93(143)117-39-15-19-67(117)89(139)107-57(81(131)103-47-73(99)125)17-11-13-37-101-75(127)21-9-7-8-10-22-76(128)102-38-14-12-18-58(82(132)104-48-74(100)126)108-90(140)68-20-16-40-118(68)94(144)66-50-148-146-42-36-78(130)106-62(44-54-25-29-56(120)30-26-54)88(138)116-80(52(4)6-2)92(142)110-60(32-34-70(96)122)84(134)112-64(46-72(98)124)86(136)114-66/h23-30,51-52,57-68,79-80,119-120H,5-22,31-50H2,1-4H3,(H2,95,121)(H2,96,122)(H2,97,123)(H2,98,124)(H2,99,125)(H2,100,126)(H,101,127)(H,102,128)(H,103,131)(H,104,132)(H,105,129)(H,106,130)(H,107,139)(H,108,140)(H,109,141)(H,110,142)(H,111,133)(H,112,134)(H,113,135)(H,114,136)(H,115,137)(H,116,138)/t51-,52-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,79-,80-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a |
Institute of Neuroscience
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor high affinity site expressed in HEK293 cells coexpressing Rluc8-tagged Galphaq, N-terminal GFP-tagged Gga... |
J Med Chem 59: 7152-66 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00564
BindingDB Entry DOI: 10.7270/Q2QN6B94 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240672

(CHEMBL4095930)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCCCC1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C44H67N11O12S2/c1-5-24(4)37-43(66)50-27(13-14-33(45)57)39(62)51-30(20-34(46)58)40(63)53-31(22-69-68-17-15-36(60)49-29(41(64)54-37)19-25-9-11-26(56)12-10-25)44(67)55-16-7-6-8-32(55)42(65)52-28(18-23(2)3)38(61)48-21-35(47)59/h9-12,23-24,27-32,37,56H,5-8,13-22H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,60)(H,50,66)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t24-,27-,28-,29-,30-,31-,32?,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240644

(CHEMBL3277916)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C40H61N11O12S2/c1-5-21(4)34-40(63)48-24(10-11-29(41)53)37(60)49-27(16-30(42)54)38(61)50-28(36(59)45-18-33(57)47-25(14-20(2)3)35(58)44-17-31(43)55)19-65-64-13-12-32(56)46-26(39(62)51-34)15-22-6-8-23(52)9-7-22/h6-9,20-21,24-28,34,52H,5,10-19H2,1-4H3,(H2,41,53)(H2,42,54)(H2,43,55)(H,44,58)(H,45,59)(H,46,56)(H,47,57)(H,48,63)(H,49,60)(H,50,61)(H,51,62)/t21-,24-,25-,26-,27-,28-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240607

(CHEMBL4093931)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCC(=O)NC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C44H66N12O14/c1-5-22(4)37-43(69)51-26(10-11-32(45)59)39(65)52-29(17-33(46)60)40(66)54-30(18-48-35(62)12-13-36(63)50-28(41(67)55-37)15-23-6-8-24(57)9-7-23)44(70)56-20-25(58)16-31(56)42(68)53-27(14-21(2)3)38(64)49-19-34(47)61/h6-9,21-22,25-31,37,57-58H,5,10-20H2,1-4H3,(H2,45,59)(H2,46,60)(H2,47,61)(H,48,62)(H,49,64)(H,50,63)(H,51,69)(H,52,65)(H,53,68)(H,54,66)(H,55,67)/t22-,25+,26-,27-,28-,29-,30-,31-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240629
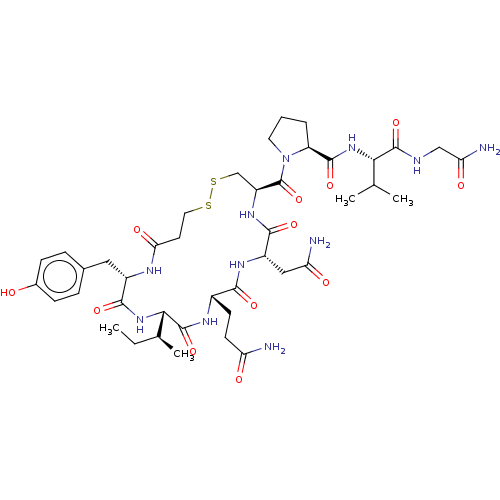
(CHEMBL4084223)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C42H63N11O12S2/c1-5-22(4)35-41(64)48-25(12-13-30(43)55)36(59)49-27(18-31(44)56)37(60)50-28(20-67-66-16-14-33(58)47-26(38(61)52-35)17-23-8-10-24(54)11-9-23)42(65)53-15-6-7-29(53)39(62)51-34(21(2)3)40(63)46-19-32(45)57/h8-11,21-22,25-29,34-35,54H,5-7,12-20H2,1-4H3,(H2,43,55)(H2,44,56)(H2,45,57)(H,46,63)(H,47,58)(H,48,64)(H,49,59)(H,50,60)(H,51,62)(H,52,61)/t22-,25-,26-,27-,28-,29-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240663
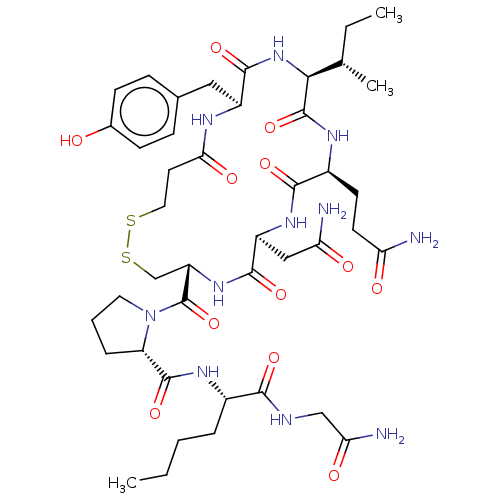
(CHEMBL4078856)Show SMILES CCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H65N11O12S2/c1-4-6-8-26(37(60)47-21-34(46)58)49-41(64)31-9-7-17-54(31)43(66)30-22-68-67-18-16-35(59)48-28(19-24-10-12-25(55)13-11-24)40(63)53-36(23(3)5-2)42(65)50-27(14-15-32(44)56)38(61)51-29(20-33(45)57)39(62)52-30/h10-13,23,26-31,36,55H,4-9,14-22H2,1-3H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,60)(H,48,59)(H,49,64)(H,50,65)(H,51,61)(H,52,62)(H,53,63)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044754

(CHEMBL3353955)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCc1ccncc1)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C47H68N12O12S2/c1-5-27(4)41-46(70)55-31(10-11-36(48)61)43(67)56-34(22-37(49)62)44(68)57-35(25-73-72-19-15-39(64)53-33(45(69)58-41)21-29-6-8-30(60)9-7-29)47(71)59(18-14-28-12-16-51-17-13-28)24-40(65)54-32(20-26(2)3)42(66)52-23-38(50)63/h6-9,12-13,16-17,26-27,31-35,41,60H,5,10-11,14-15,18-25H2,1-4H3,(H2,48,61)(H2,49,62)(H2,50,63)(H,52,66)(H,53,64)(H,54,65)(H,55,70)(H,56,67)(H,57,68)(H,58,69)/t27-,31-,32-,33-,34-,35-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044727

(CHEMBL3354592)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSCC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1cccc(C)c1)[C@@H](C)CC |r| Show InChI InChI=1S/C49H71N11O12S/c1-6-29(5)43-48(71)56-33(14-15-38(50)62)45(68)58-37(23-39(51)63)46(69)57-34(16-18-73-19-17-41(65)54-36(47(70)59-43)22-30-10-12-32(61)13-11-30)49(72)60(25-31-9-7-8-28(4)21-31)26-42(66)55-35(20-27(2)3)44(67)53-24-40(52)64/h7-13,21,27,29,33-37,43,61H,6,14-20,22-26H2,1-5H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,66)(H,56,71)(H,57,69)(H,58,68)(H,59,70)/t29-,33-,34-,35-,36-,37-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044750
(CHEMBL3354571)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCc1cccs1)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C46H67N11O12S3/c1-5-26(4)40-45(68)53-30(12-13-35(47)59)42(65)54-33(21-36(48)60)43(66)55-34(24-72-71-18-15-38(62)51-32(44(67)56-40)20-27-8-10-28(58)11-9-27)46(69)57(16-14-29-7-6-17-70-29)23-39(63)52-31(19-25(2)3)41(64)50-22-37(49)61/h6-11,17,25-26,30-34,40,58H,5,12-16,18-24H2,1-4H3,(H2,47,59)(H2,48,60)(H2,49,61)(H,50,64)(H,51,62)(H,52,63)(H,53,68)(H,54,65)(H,55,66)(H,56,67)/t26-,30-,31-,32-,33-,34-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240673
(CHEMBL4096848)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C40H59N11O12S2/c1-4-20(2)33-39(62)47-24(11-12-29(41)53)35(58)48-26(17-30(42)54)36(59)49-27(40(63)51-14-5-6-28(51)38(61)45-21(3)34(57)44-18-31(43)55)19-65-64-15-13-32(56)46-25(37(60)50-33)16-22-7-9-23(52)10-8-22/h7-10,20-21,24-28,33,52H,4-6,11-19H2,1-3H3,(H2,41,53)(H2,42,54)(H2,43,55)(H,44,57)(H,45,61)(H,46,56)(H,47,62)(H,48,58)(H,49,59)(H,50,60)/t20-,21-,24-,25-,26-,27-,28-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044686
(CHEMBL3354594)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1)[C@@H](C)CC |r| Show InChI InChI=1S/C48H68FN11O12S/c1-5-27(4)42-47(71)56-32(16-17-37(50)62)44(68)57-35(21-38(51)63)45(69)58-36(25-73-18-6-7-40(65)54-34(46(70)59-42)20-28-10-14-31(61)15-11-28)48(72)60(23-29-8-12-30(49)13-9-29)24-41(66)55-33(19-26(2)3)43(67)53-22-39(52)64/h8-15,26-27,32-36,42,61H,5-7,16-25H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,66)(H,56,71)(H,57,68)(H,58,69)(H,59,70)/t27-,32-,33-,34-,35-,36-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human OTR expressed in HEK293 cells assessed as increase in intracellular calcium level measured at 3 secs interval f... |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240675
(CHEMBL4101771)Show SMILES CCCCCCC(NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C45H69N11O12S2/c1-4-6-7-8-10-28(39(62)49-23-36(48)60)51-43(66)33-11-9-19-56(33)45(68)32-24-70-69-20-18-37(61)50-30(21-26-12-14-27(57)15-13-26)42(65)55-38(25(3)5-2)44(67)52-29(16-17-34(46)58)40(63)53-31(22-35(47)59)41(64)54-32/h12-15,25,28-33,38,57H,4-11,16-24H2,1-3H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,62)(H,50,61)(H,51,66)(H,52,67)(H,53,63)(H,54,64)(H,55,65)/t25-,28?,29-,30-,31-,32-,33-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240631
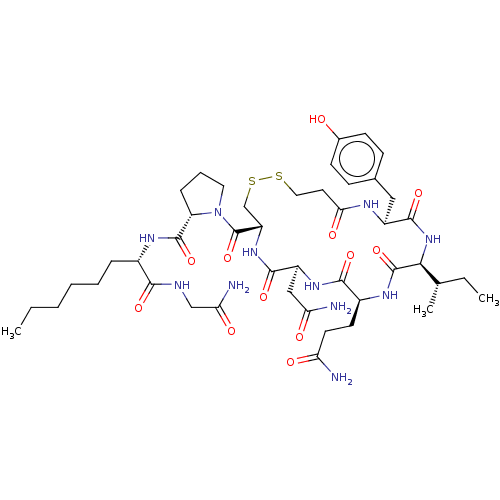
(CHEMBL4065273)Show SMILES CCCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C45H69N11O12S2/c1-4-6-7-8-10-28(39(62)49-23-36(48)60)51-43(66)33-11-9-19-56(33)45(68)32-24-70-69-20-18-37(61)50-30(21-26-12-14-27(57)15-13-26)42(65)55-38(25(3)5-2)44(67)52-29(16-17-34(46)58)40(63)53-31(22-35(47)59)41(64)54-32/h12-15,25,28-33,38,57H,4-11,16-24H2,1-3H3,(H2,46,58)(H2,47,59)(H2,48,60)(H,49,62)(H,50,61)(H,51,66)(H,52,67)(H,53,63)(H,54,64)(H,55,65)/t25-,28-,29-,30-,31-,32-,33-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523555
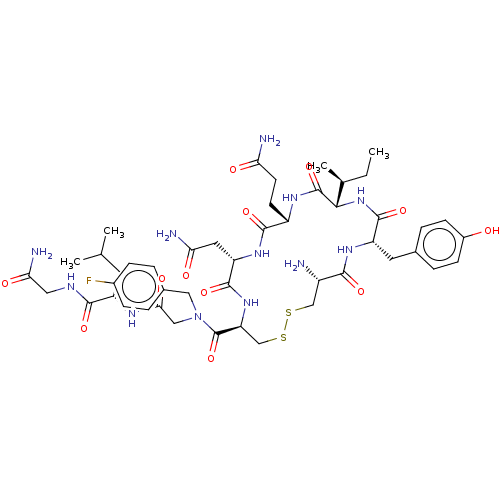
(CHEMBL4474284)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)Cc1ccc(F)cc1 |r| Show InChI InChI=1S/C47H67FN12O12S2/c1-5-25(4)40-46(71)55-31(14-15-36(50)62)43(68)57-34(18-37(51)63)44(69)58-35(23-74-73-22-30(49)41(66)56-33(45(70)59-40)17-26-8-12-29(61)13-9-26)47(72)60(20-27-6-10-28(48)11-7-27)21-39(65)54-32(16-24(2)3)42(67)53-19-38(52)64/h6-13,24-25,30-35,40,61H,5,14-23,49H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,71)(H,56,66)(H,57,68)(H,58,69)(H,59,70)/t25-,30-,31-,32-,33-,34-,35-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human OTR expressed in HEK293 cells assessed as increase in intracellular calcium level measured at 3 secs interval f... |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240628
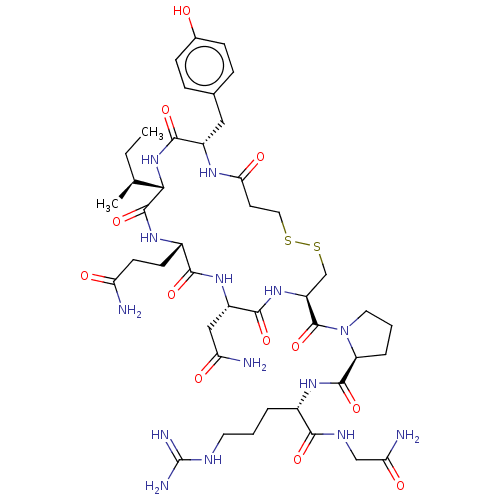
(CHEMBL4094454)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N14O12S2/c1-3-22(2)35-41(68)53-26(12-13-31(44)59)37(64)54-28(19-32(45)60)38(65)55-29(21-71-70-17-14-34(62)51-27(39(66)56-35)18-23-8-10-24(58)11-9-23)42(69)57-16-5-7-30(57)40(67)52-25(6-4-15-49-43(47)48)36(63)50-20-33(46)61/h8-11,22,25-30,35,58H,3-7,12-21H2,1-2H3,(H2,44,59)(H2,45,60)(H2,46,61)(H,50,63)(H,51,62)(H,52,67)(H,53,68)(H,54,64)(H,55,65)(H,56,66)(H4,47,48,49)/t22-,25-,26-,27-,28-,29-,30-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044768
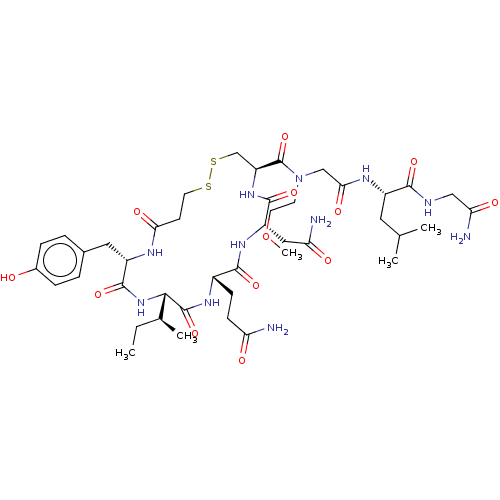
(CHEMBL3353942)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCOC)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C43H67N11O13S2/c1-6-24(4)37-42(65)50-27(11-12-32(44)56)39(62)51-30(19-33(45)57)40(63)52-31(22-69-68-16-13-35(59)48-29(41(64)53-37)18-25-7-9-26(55)10-8-25)43(66)54(14-15-67-5)21-36(60)49-28(17-23(2)3)38(61)47-20-34(46)58/h7-10,23-24,27-31,37,55H,6,11-22H2,1-5H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,61)(H,48,59)(H,49,60)(H,50,65)(H,51,62)(H,52,63)(H,53,64)/t24-,27-,28-,29-,30-,31-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50523557
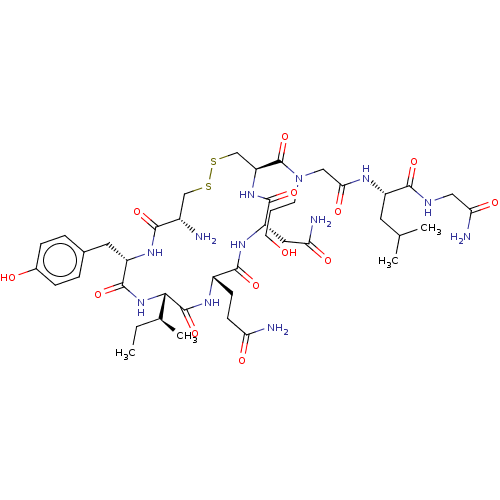
(CHEMBL4583231)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCCO)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H68N12O13S2/c1-5-23(4)36-42(67)50-27(11-12-32(45)58)39(64)52-30(17-33(46)59)40(65)53-31(21-70-69-20-26(44)37(62)51-29(41(66)54-36)16-24-7-9-25(57)10-8-24)43(68)55(13-6-14-56)19-35(61)49-28(15-22(2)3)38(63)48-18-34(47)60/h7-10,22-23,26-31,36,56-57H,5-6,11-21,44H2,1-4H3,(H2,45,58)(H2,46,59)(H2,47,60)(H,48,63)(H,49,61)(H,50,67)(H,51,62)(H,52,64)(H,53,65)(H,54,66)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human OTR expressed in HEK293 cells assessed as increase in intracellular calcium level measured at 3 secs interval f... |
J Med Chem 62: 3297-3310 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01691
BindingDB Entry DOI: 10.7270/Q2GF0XXQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50240604

(CHEMBL4092971)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCC(=O)NC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1C[C@H](O)C[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NNC(N)=O |r| Show InChI InChI=1S/C43H65N13O14/c1-5-21(4)35-41(68)49-25(10-11-31(44)59)36(63)50-28(17-32(45)60)37(64)52-29(18-47-33(61)12-13-34(62)48-27(38(65)53-35)15-22-6-8-23(57)9-7-22)42(69)56-19-24(58)16-30(56)40(67)51-26(14-20(2)3)39(66)54-55-43(46)70/h6-9,20-21,24-30,35,57-58H,5,10-19H2,1-4H3,(H2,44,59)(H2,45,60)(H,47,61)(H,48,62)(H,49,68)(H,50,63)(H,51,67)(H,52,64)(H,53,65)(H,54,66)(H3,46,55,70)/t21-,24+,25-,26-,27-,28-,29-,30-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as calcium mobilization measured after 30 mins by Fluo-4-AM dye based FLIPR assay |
Bioorg Med Chem Lett 27: 2331-2335 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.030
BindingDB Entry DOI: 10.7270/Q2BR8V9Q |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044683

(CHEMBL3354597)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N(CCc1ccccc1)CC(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC |r| Show InChI InChI=1S/C49H71N11O12S/c1-5-29(4)43-48(71)56-33(17-18-38(50)62)45(68)57-36(24-39(51)63)46(69)58-37(27-73-21-9-12-41(65)54-35(47(70)59-43)23-31-13-15-32(61)16-14-31)49(72)60(20-19-30-10-7-6-8-11-30)26-42(66)55-34(22-28(2)3)44(67)53-25-40(52)64/h6-8,10-11,13-16,28-29,33-37,43,61H,5,9,12,17-27H2,1-4H3,(H2,50,62)(H2,51,63)(H2,52,64)(H,53,67)(H,54,65)(H,55,66)(H,56,71)(H,57,68)(H,58,69)(H,59,70)/t29-,33-,34-,35-,36-,37-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in CHO-K1 cells after 5 hrs by firefly luciferase reporter gene assay |
J Med Chem 57: 5306-17 (2014)
Article DOI: 10.1021/jm500365s
BindingDB Entry DOI: 10.7270/Q2WD426V |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50044676

(CHEMBL439044)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O)[C@@H](C)CC Show InChI InChI=1S/C43H65N11O12S2/c1-5-23(4)36-42(65)49-26(12-13-32(44)56)38(61)50-29(19-33(45)57)39(62)52-30(21-68-67-16-14-35(59)48-28(40(63)53-36)18-24-8-10-25(55)11-9-24)43(66)54-15-6-7-31(54)41(64)51-27(17-22(2)3)37(60)47-20-34(46)58/h8-11,22-23,26-31,36,55H,5-7,12-21H2,1-4H3,(H2,44,56)(H2,45,57)(H2,46,58)(H,47,60)(H,48,59)(H,49,65)(H,50,61)(H,51,64)(H,52,62)(H,53,63)/t23-,26-,27-,28-,29-,30-,31-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human OTR expressed in CHO cells assessed as increase in calcium flux after 60 to 120 mins by fluo-4 dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2017.12.027
BindingDB Entry DOI: 10.7270/Q2VT1VPB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































