

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409523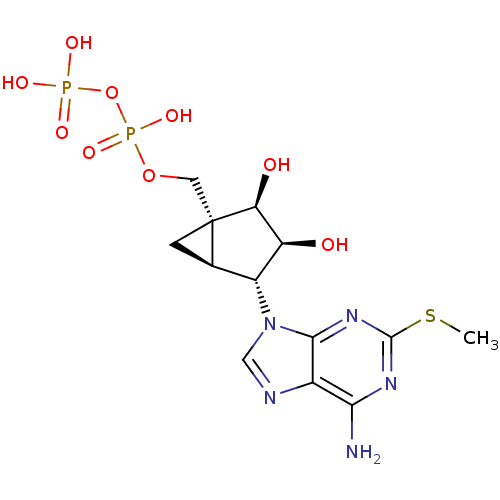 (CHEMBL2112093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50409523 (CHEMBL2112093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Accumulation of inositol phosphate in 1321N1 astrocytoma cells expressing human P2Y1 purinoceptor | J Med Chem 45: 2090-100 (2002) BindingDB Entry DOI: 10.7270/Q25H7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50118232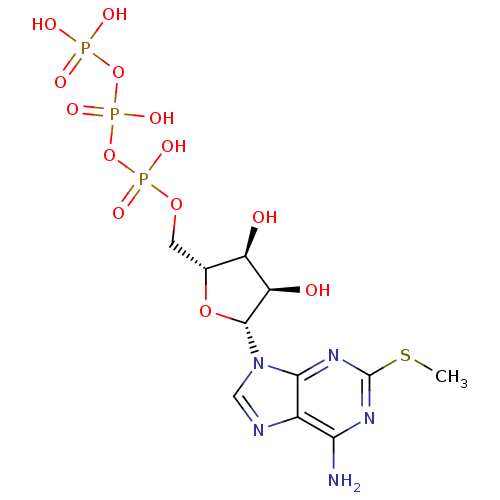 (2-MeSATP | ATP, 2-meS | CHEMBL336208) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50409654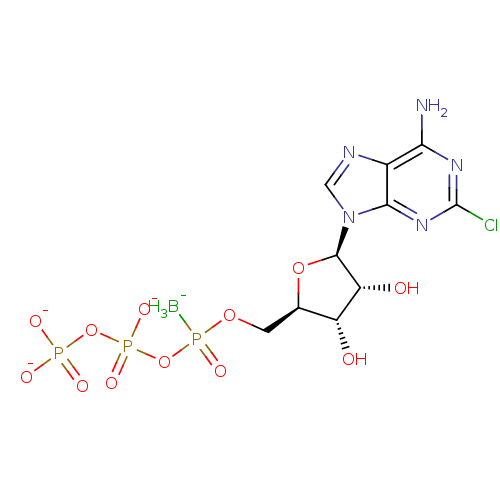 (CHEMBL2021421) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50410189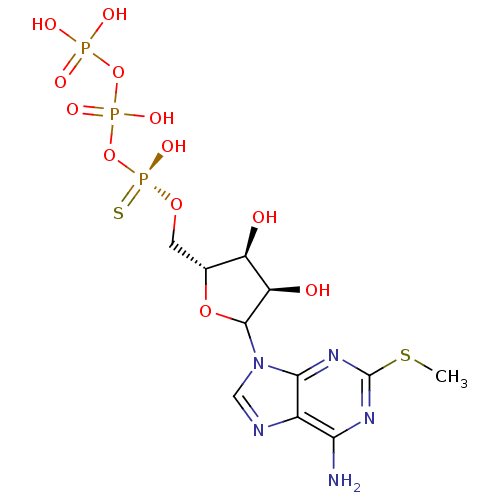 (CHEMBL2093075) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 2 (RAT) | BDBM50118243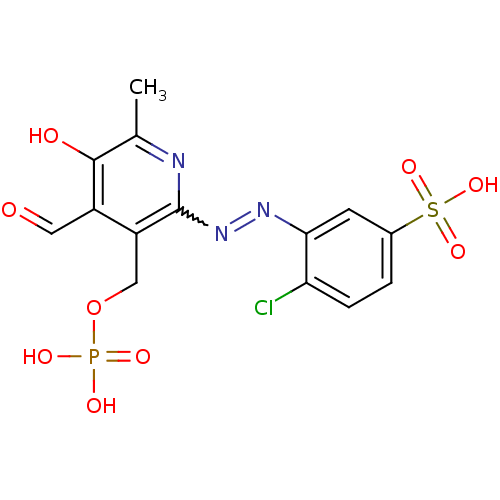 (CHEMBL334823 | MRS 2191) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat receptor P2X purinoceptor 2 (P2X2) at 10 uM, expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242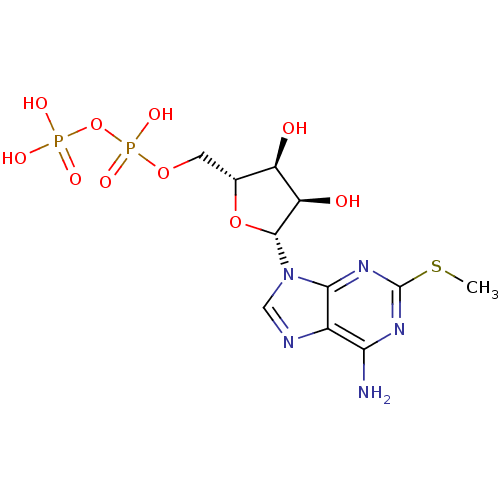 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of wild-type P2Y1 receptors expressed in COS-7 cells for the accumulation of inositol phosphate | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118215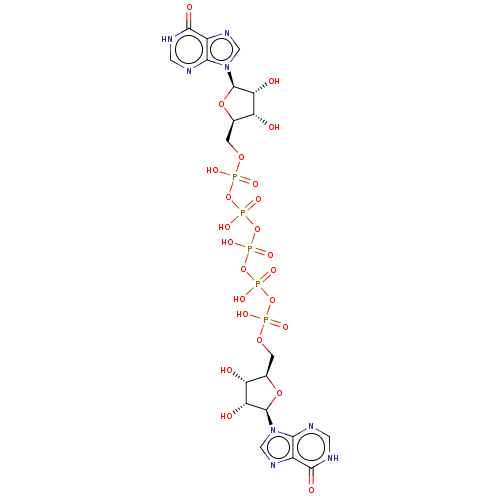 (CHEMBL1628528 | Ip5I) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human S317A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50308552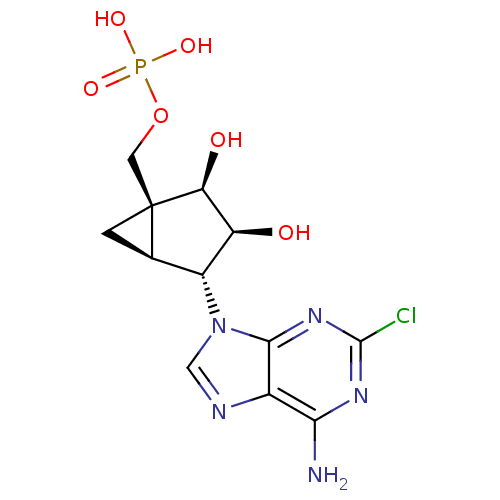 ((1'S,2'R,3'S,4'R,5'S)-4-(6-Amino-2-chloro-9H-purin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a |
National Institutes of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y1 receptor expressed in human 1321N1 cells by PLC assay | J Med Chem 53: 2562-76 (2010) Article DOI: 10.1021/jm9018542 BindingDB Entry DOI: 10.7270/Q20C4VVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was measured for the antagonism of the activation of phospholipase C in mutant(WT) human P2Y1 receptor. | J Med Chem 41: 1456-66 (1998) Article DOI: 10.1021/jm970684u BindingDB Entry DOI: 10.7270/Q2TM7BSC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was measured for the antagonism of the activation of phospholipase C in mutant(S317A) human P2Y1 receptor. | J Med Chem 41: 1456-66 (1998) Article DOI: 10.1021/jm970684u BindingDB Entry DOI: 10.7270/Q2TM7BSC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50370180 (CHEMBL607612) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422407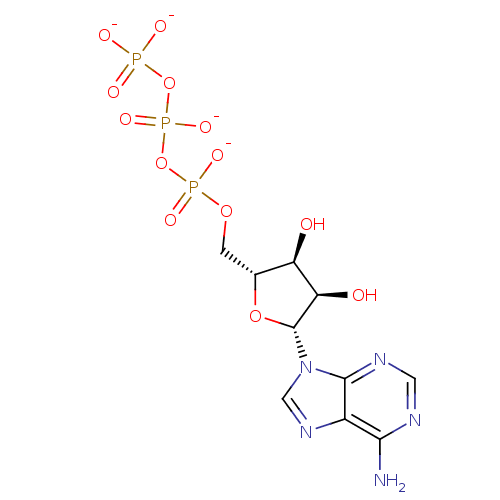 (CHEMBL2364735) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (Homo sapiens (Human)) | BDBM50118219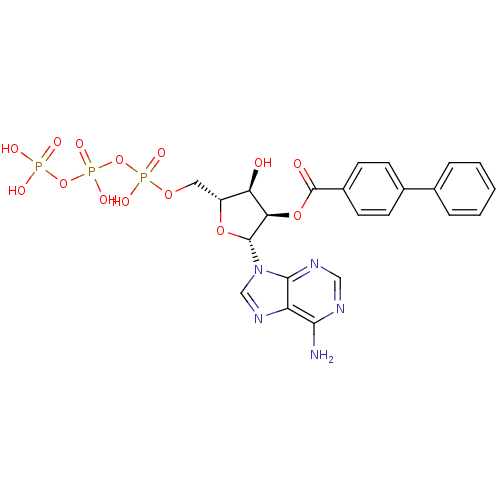 (Bz-ATP | CHEMBL339386) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant human P2X purinoceptor 1 (P2X1 ) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of wild-type P2Y1 receptors expressed in COS-7 cells for the accumulation of inositol phosphate i... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at G-protein coupled P2Y1 receptor expressed in human 1321N1 cells assessed as increase in calcium by Fura2 assay | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was measured for the antagonism of the activation of phospholipase C in mutant(T221A) human P2Y1 receptor. | J Med Chem 41: 1456-66 (1998) Article DOI: 10.1021/jm970684u BindingDB Entry DOI: 10.7270/Q2TM7BSC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human K196A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50267999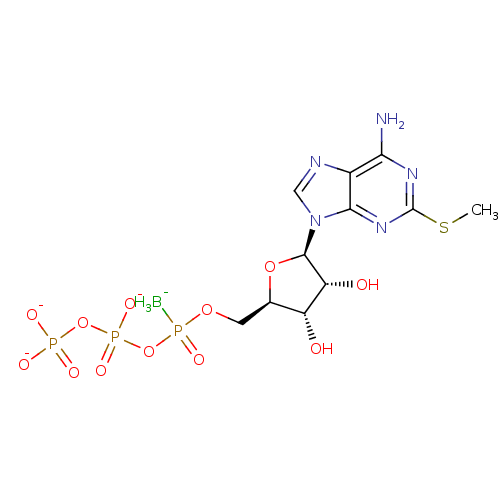 (({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50398072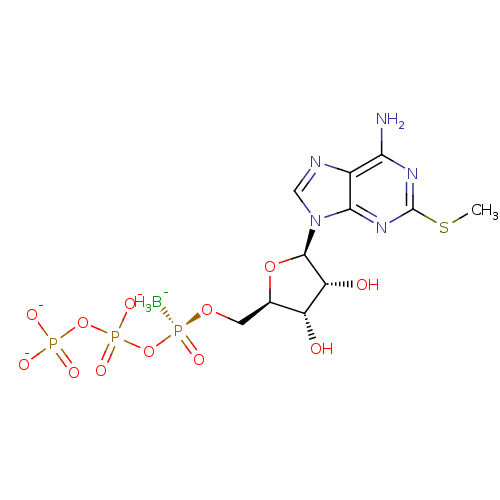 (CHEMBL2181943) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at rat P2Y1R expressed in HEK293 cells assessed as release of intracellular calcium by fluorescence based assay | J Med Chem 55: 7623-35 (2012) Article DOI: 10.1021/jm3006355 BindingDB Entry DOI: 10.7270/Q2KK9CXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50423774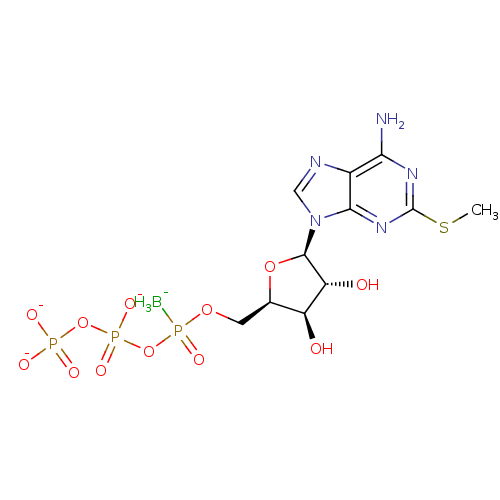 (CHEMBL2309024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor expressed in human HEK293 cells | J Med Chem 53: 2472-81 (2010) Article DOI: 10.1021/jm901621h BindingDB Entry DOI: 10.7270/Q2DN4565 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human R301A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50267999 (({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50267999 (({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50195835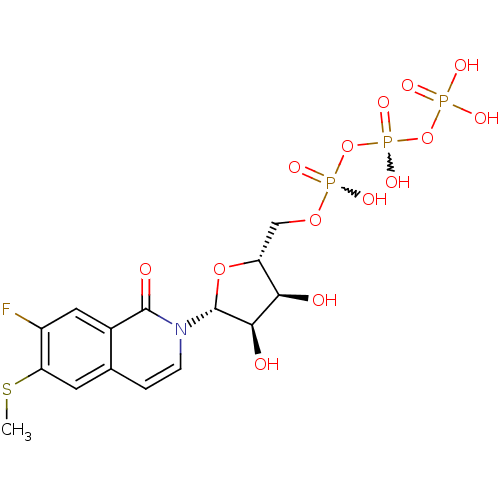 (({[({[(2R,3S,4R,5R)-5-[7-fluoro-6-(methylsulfanyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 562-5 (2007) Article DOI: 10.1016/j.bmcl.2006.09.017 BindingDB Entry DOI: 10.7270/Q2CC11JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50197695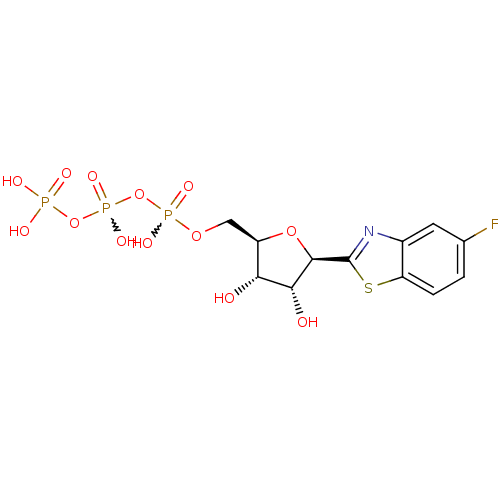 (({[({[(2R,3S,4R,5R)-5-(5-fluoro-1,3-benzothiazol-2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 558-61 (2007) Article DOI: 10.1016/j.bmcl.2006.10.038 BindingDB Entry DOI: 10.7270/Q2QN67MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50112757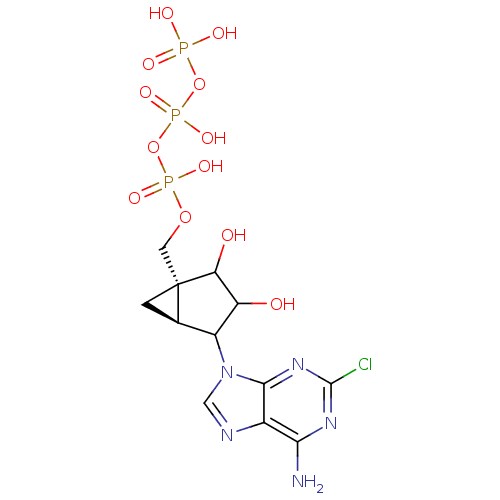 (CHEMBL66627 | triPhosphoric acid -[4-(6-amino-2-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Activation of Purinoceptor P2Y1-mediated phospholipase C in turkey erythrocyte membranes | J Med Chem 45: 2090-100 (2002) BindingDB Entry DOI: 10.7270/Q25H7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration to activate wild-type P2Y1 receptor expressed in COS-7 cells for the accumulation of inositol phosphate | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human E209D mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422406 (2-Chloroadenosine Triphosphate Tetrasodium | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human D208A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50112763 (CHEMBL303596 | triPhosphoric acid -[4-(6-amino-2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Activation of Purinoceptor P2Y1-mediated phospholipase C in turkey erythrocyte membranes | J Med Chem 45: 2090-100 (2002) BindingDB Entry DOI: 10.7270/Q25H7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human D289A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50195845 (({[({[(2R,3S,4R,5R)-5-[6-(dimethylamino)-7-fluoro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 562-5 (2007) Article DOI: 10.1016/j.bmcl.2006.09.017 BindingDB Entry DOI: 10.7270/Q2CC11JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50370496 (CHEMBL610595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422408 (CHEMBL2364736) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50370496 (CHEMBL610595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Concentration required for calcium mobilization at rat purinergic 2Y1 receptor expressed in HEK 293 cells | J Med Chem 47: 4405-16 (2004) Article DOI: 10.1021/jm049771u BindingDB Entry DOI: 10.7270/Q2PC334S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50435007 (CHEMBL2386493) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at rat brain P2Y1R transfected in HEK293 cells assessed as induction of intracellular calcium mobilization by fluorescence assay | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human R195A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human E209R mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50195825 (({[({[(2R,3S,4R,5R)-5-(7-fluoro-1-oxo-1,2-dihydroi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 562-5 (2007) Article DOI: 10.1016/j.bmcl.2006.09.017 BindingDB Entry DOI: 10.7270/Q2CC11JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50195825 (({[({[(2R,3S,4R,5R)-5-(7-fluoro-1-oxo-1,2-dihydroi...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 558-61 (2007) Article DOI: 10.1016/j.bmcl.2006.10.038 BindingDB Entry DOI: 10.7270/Q2QN67MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50118222 (MRS 2257) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 1 (P2X1) at 1 uM,expressed in Xenopus oocytes | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50118213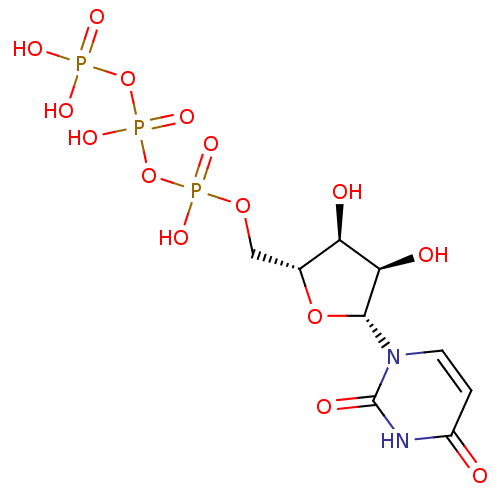 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at wild type P2Y2 receptor (unknown origin) expressed in human 1321N1 astrocytoma cells assessed as increase in intracellular calciu... | J Med Chem 60: 8425-8440 (2017) Article DOI: 10.1021/acs.jmedchem.7b00854 BindingDB Entry DOI: 10.7270/Q29P33TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration required for the activation of human K125A mutant strain P2Y1 receptors expressed in COS-7 cells for the accumulation of inos... | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 2 (Homo sapiens (Human)) | BDBM50195847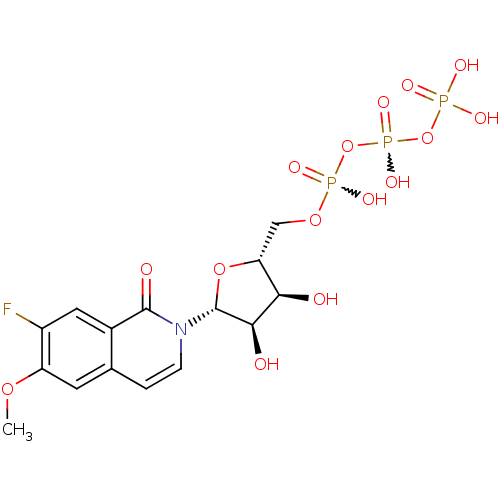 (({[({[(2R,3S,4R,5R)-5-(7-fluoro-6-methoxy-1-oxo-1,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
UCB-Group Curated by ChEMBL | Assay Description Agonist activity at human P2Y2 receptor expressed in human 1321N1 by FLIPR assay | Bioorg Med Chem Lett 17: 562-5 (2007) Article DOI: 10.1016/j.bmcl.2006.09.017 BindingDB Entry DOI: 10.7270/Q2CC11JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50112767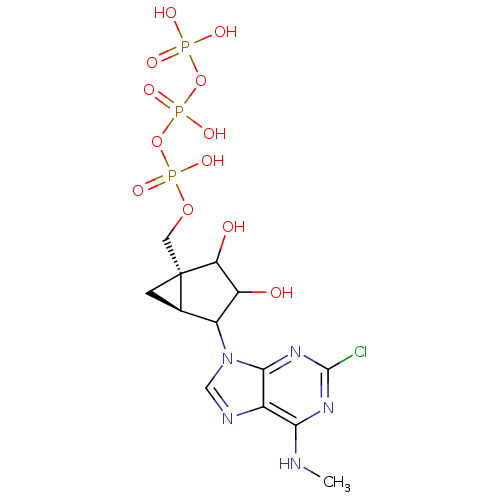 (CHEMBL291608 | triPhosphoric acid -[2,3-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Activation of Purinoceptor P2Y1-mediated phospholipase C in turkey erythrocyte membranes | J Med Chem 45: 2090-100 (2002) BindingDB Entry DOI: 10.7270/Q25H7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Effective concentration to activate human Y273F mutant strain P2Y1 receptor expressed in COS-7 cells for the accumulation of inositol phosphate | J Med Chem 47: 5393-404 (2004) Article DOI: 10.1021/jm049914c BindingDB Entry DOI: 10.7270/Q2GQ6ZJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 1 (Homo sapiens (Human)) | BDBM50370141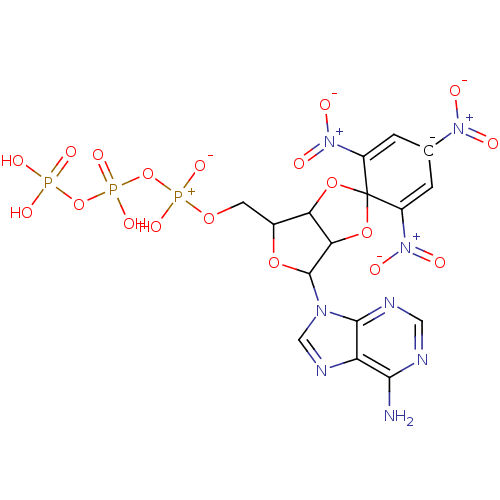 (TNP-ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against recombinant human P2X purinoceptor 1 (P2X1 ) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 674 total ) | Next | Last >> |