

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50438936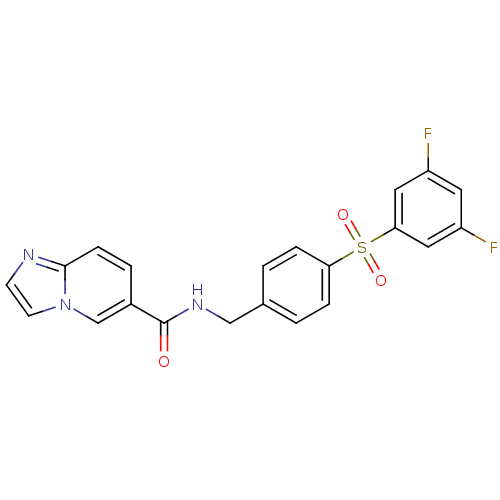 (CHEMBL2420629 | GNE-617) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in human PC3 cells assessed as reduction in NAD level after 48 hrs by mass spectrometry | Bioorg Med Chem Lett 23: 5488-97 (2013) Article DOI: 10.1016/j.bmcl.2013.08.074 BindingDB Entry DOI: 10.7270/Q2DF6SQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50011266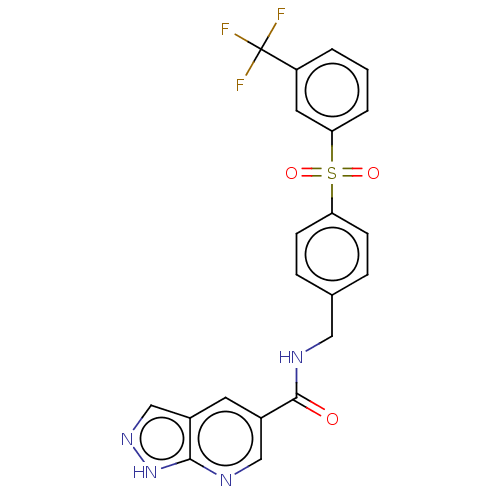 (CHEMBL3260358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in human PC3 cells assessed as reduction in NAD level after 48 hrs by mass spectrometry | Bioorg Med Chem Lett 23: 5488-97 (2013) Article DOI: 10.1016/j.bmcl.2013.08.074 BindingDB Entry DOI: 10.7270/Q2DF6SQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Mus musculus) | BDBM50438936 (CHEMBL2420629 | GNE-617) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in mouse cells assessed as reduction in NAD level after 48 hrs by mass spectrometry | Bioorg Med Chem Lett 23: 5488-97 (2013) Article DOI: 10.1016/j.bmcl.2013.08.074 BindingDB Entry DOI: 10.7270/Q2DF6SQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570832 (CHEMBL4848594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570834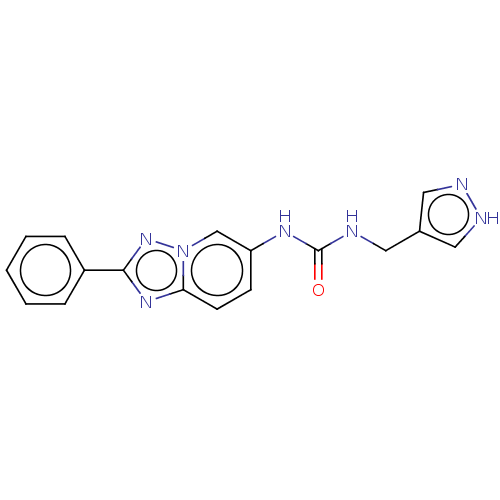 (CHEMBL4877644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569533 (CHEMBL4858231 | US11452717, Compound TableB-2.166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572570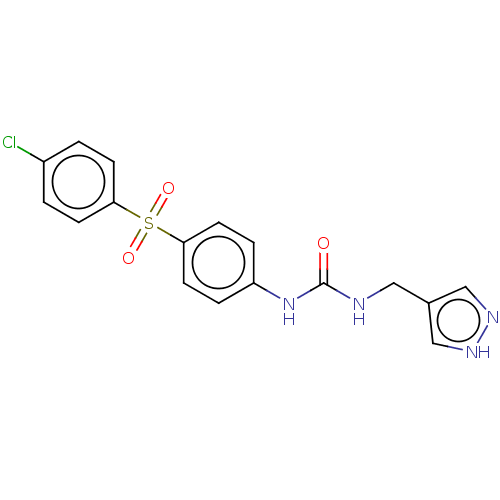 (1-[4-(4-Chloro-benzenesulfonyl)-phenyl]-3-(1H-pyra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569538 (CHEMBL4859010 | US11452717, Compound TableB-1.48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570831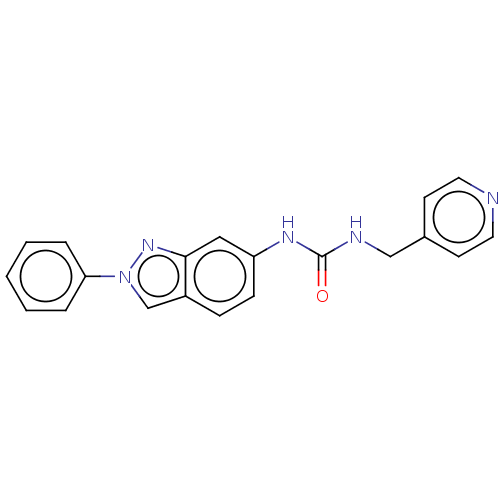 (CHEMBL4846468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569529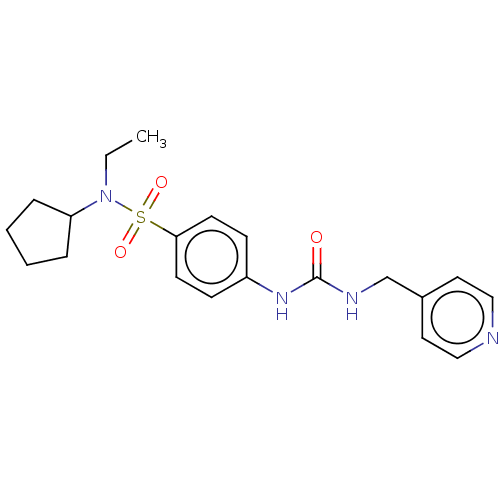 (CHEMBL4867743 | US11452717, Compound TableB-2.173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569536 (CHEMBL4852773 | US11452717, Compound TableB-2.219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570836 (CHEMBL4860172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Mus musculus) | BDBM50011266 (CHEMBL3260358) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Forma Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of NAMPT in mouse cells assessed as reduction in NAD level after 48 hrs by mass spectrometry | Bioorg Med Chem Lett 23: 5488-97 (2013) Article DOI: 10.1016/j.bmcl.2013.08.074 BindingDB Entry DOI: 10.7270/Q2DF6SQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569541 (CHEMBL4859855 | US11452717, Compound TableB-5.29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569540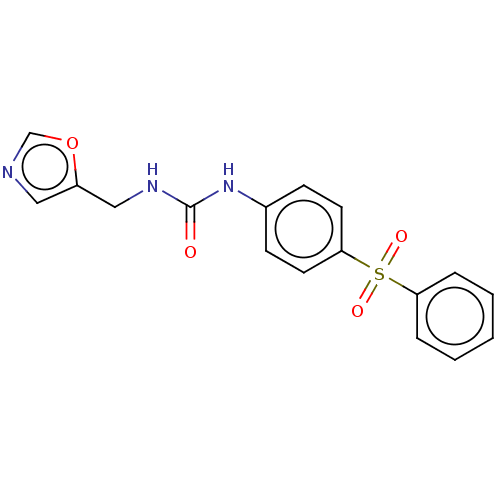 (CHEMBL4868976 | US11452717, Compound TableB-1.47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569535 (CHEMBL4856434 | US11452717, Compound TableB-2.221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570844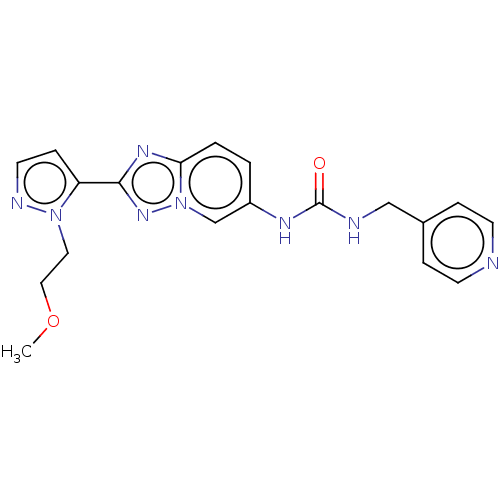 (CHEMBL4877360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50570833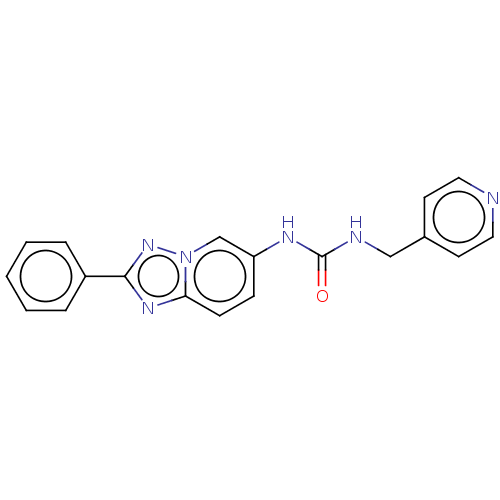 (CHEMBL4861097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT assessed as increase in NMN production by fluorescence based analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128048 BindingDB Entry DOI: 10.7270/Q27W6H07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50569534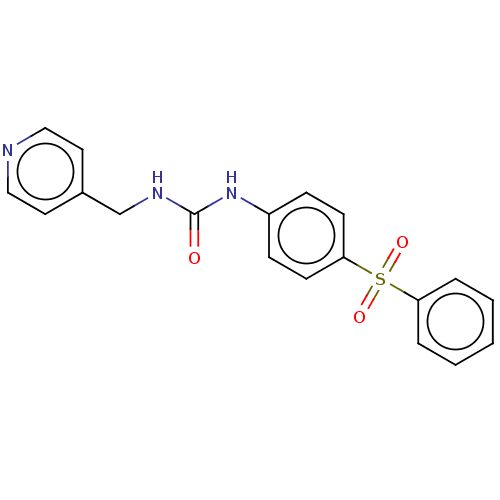 (CHEMBL4868692 | US11452717, Compound TableB-2.224) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human NAMPT | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128007 BindingDB Entry DOI: 10.7270/Q2MK6HPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572812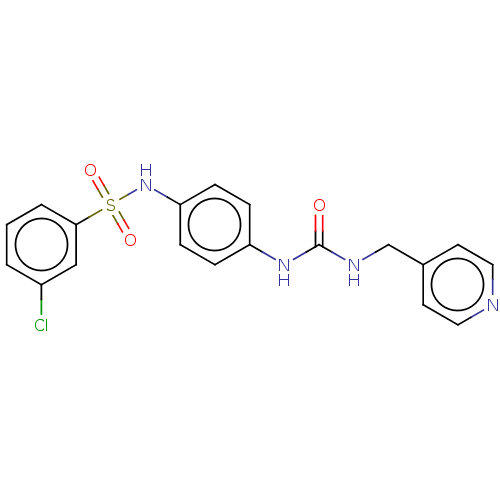 (3-Chloro-N-[4-(3-pyridin-4-ylmethyl-ureido)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572813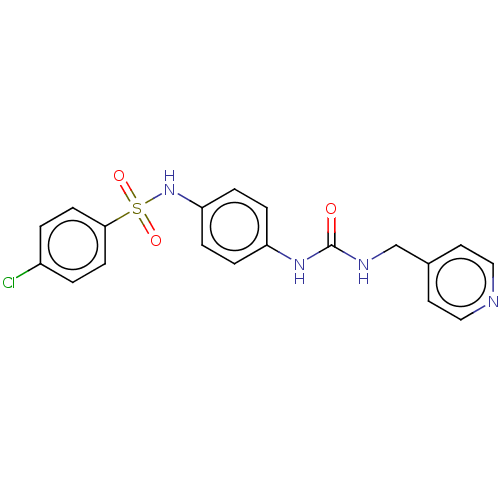 (4-Chloro-N-[4-(3-pyridin-4-ylmethyl-ureido)-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572814 (N-[4-(3-Benzyl-ureido)-phenyl]-C-phenyl-methanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572815 (1-(2-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572816 (1-(3-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572817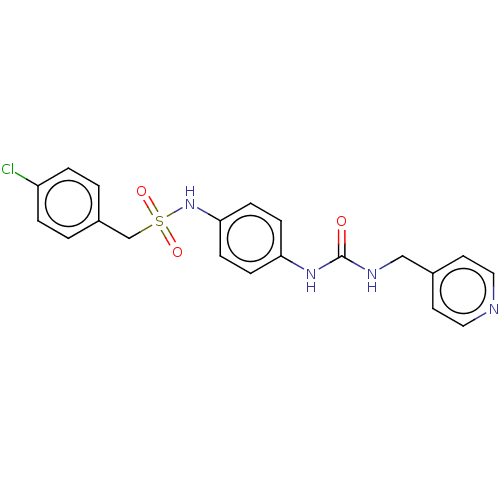 (1-(4-chlorophenyl)-N-(4-(3-(pyridin-4-ylmethyl)ure...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572818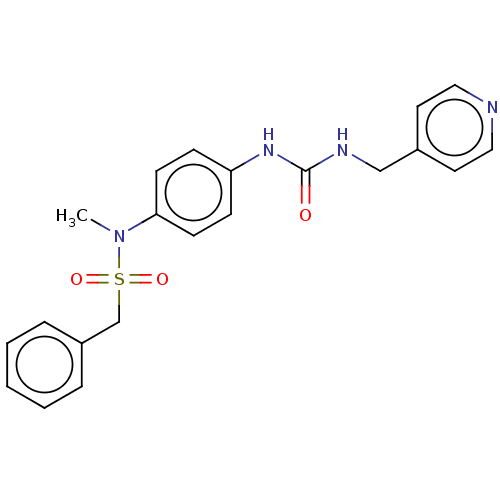 (N-Methyl-C-phenyl-N-[4-(3-pyridin-4-ylmethyl-ureid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572861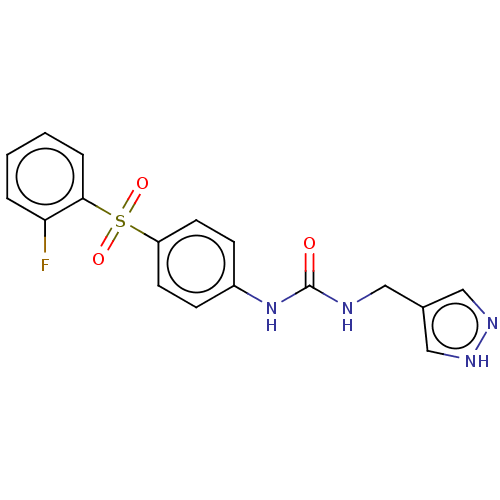 (1-((1H-Pyrazol-4-yl)methyl)-3-(4-((2-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572862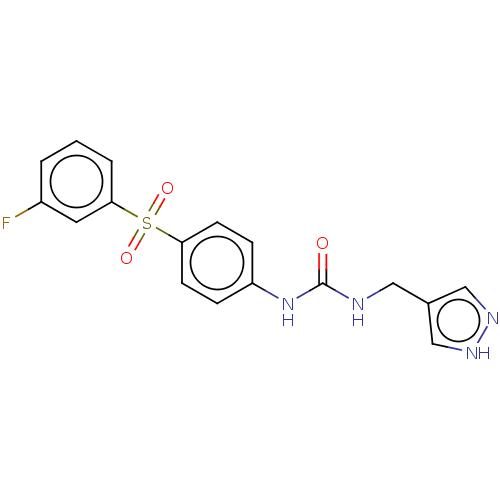 (1-((1H-Pyrazol-4-yl)methyl)-3-(4-((3-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572863 (1-((1H-Pyrazol-4-yl)methyl)-3-(4-((4-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572864 (1-((1H-Pyrazol-4-yl)methyl)-3-(4-((3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572869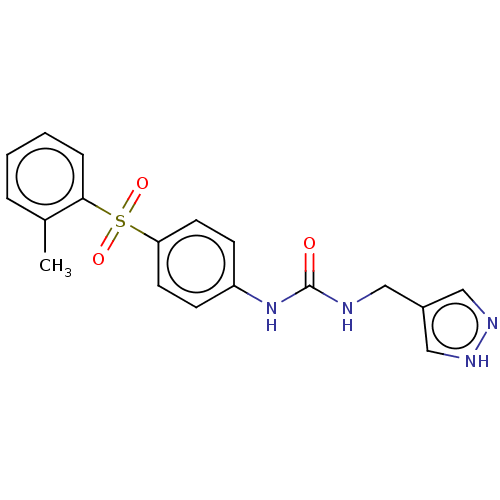 (1-(1H-Pyrazol-4-ylmethyl)-3-[4-(toluene-2-sulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572871 (1-[4-(3,4-Dichloro-benzenesulfonyl)-phenyl]-3-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572872 (C-(3-Chloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572873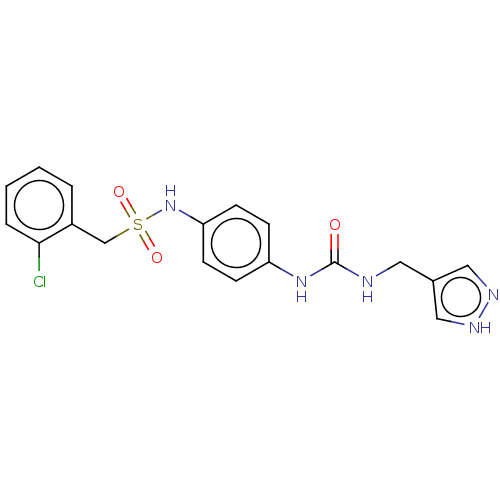 (C-(2-Chloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572874 (C-(4-Chloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572877 (C-(3,4-Dichloro-phenyl)-N-{4-[3-(1H-pyrazol-4-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572882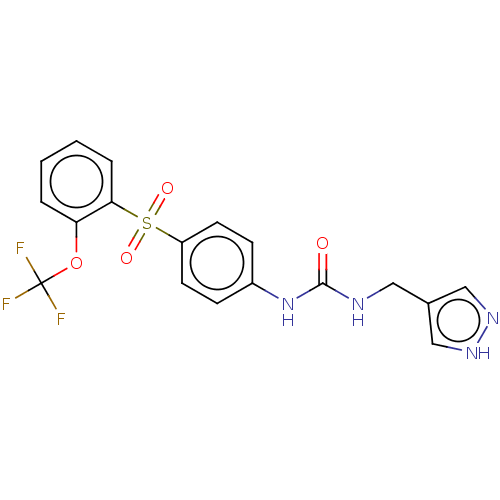 (1-(1H-Pyrazol-4-ylmethyl)-3-[4-(2-trifluoromethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572883 (1-(1H-Pyrazol-4-ylmethyl)-3-[4-(3-trifluoromethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572891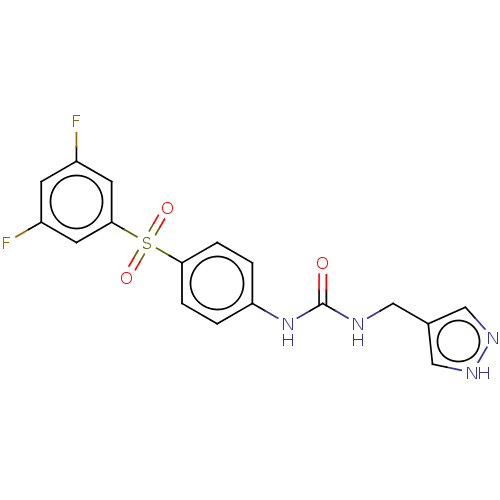 (1-[4-(3,5-Difluoro-benzenesulfonyl)-phenyl]-3-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572892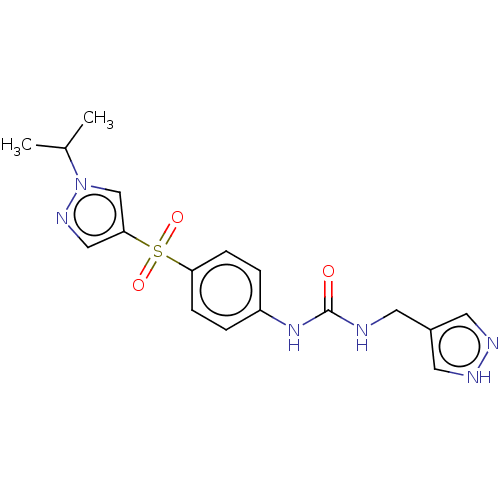 (1-[4-(1-Isopropyl-1H-pyrazole-4-sulfonyl)-phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572897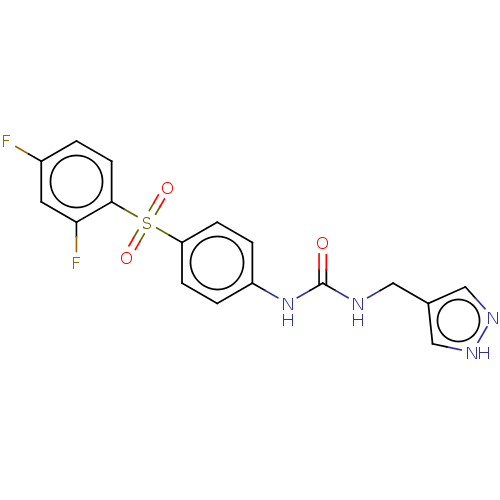 (1-[4-(2,4-Difluoro-benzenesulfonyl)-phenyl]-3-(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572929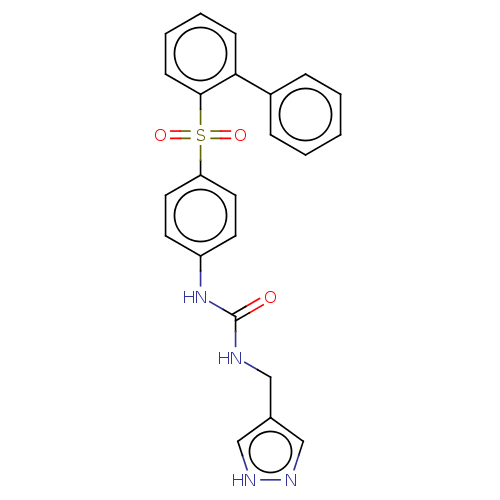 (1-((1H-Pyrazol-4-yl)methyl)-3-(4-([1,1'-biphenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572939 (1-[4-(3-Iodo-benzenesulfonyl)-phenyl]-3-(1H-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572940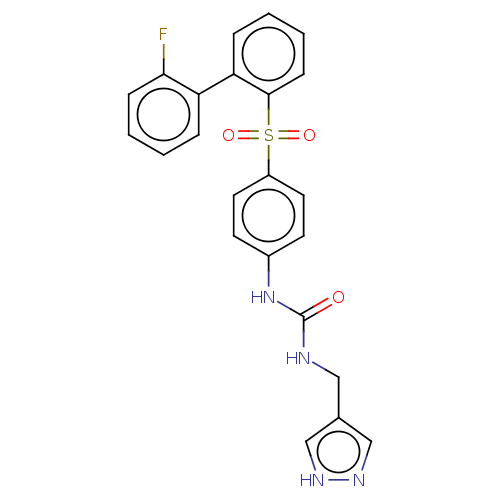 (1-[4-(2'-Fluoro-biphenyl-2-sulfonyl)-phenyl]-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572941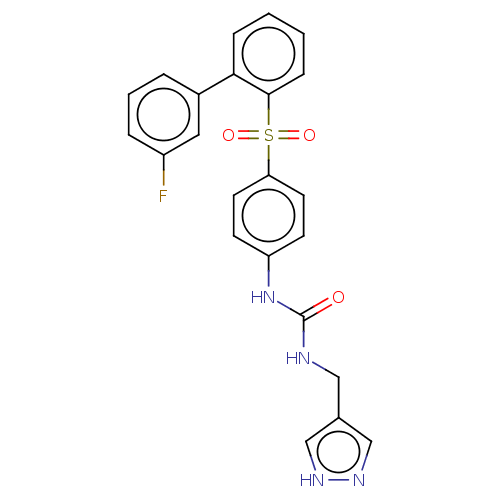 (1-[4-(3'-Fluoro-biphenyl-2-sulfonyl)-phenyl]-3-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572957 (1-(3-Chlorophenyl)-N-(4-(3-(oxazol-5-ylmethyl)urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572958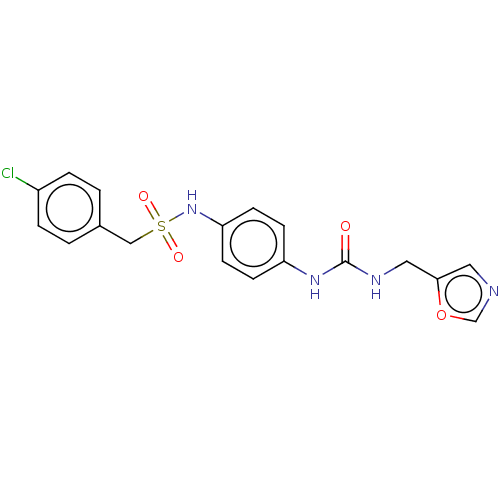 (1-(4-Chlorophenyl)-N-(4-(3-(oxazol-5-ylmethyl)urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572959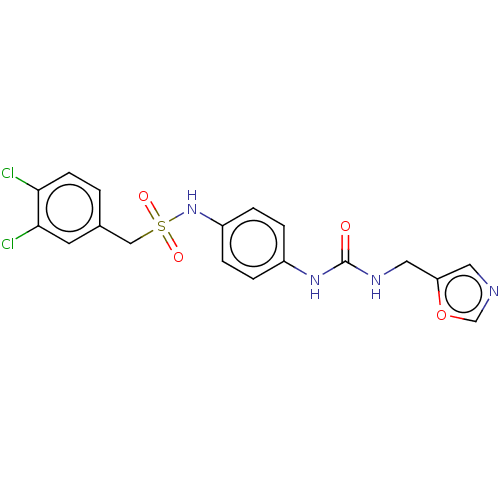 (1-(3,4-Dichlorophenyl)-N-(4-(3-(oxazol-5-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572960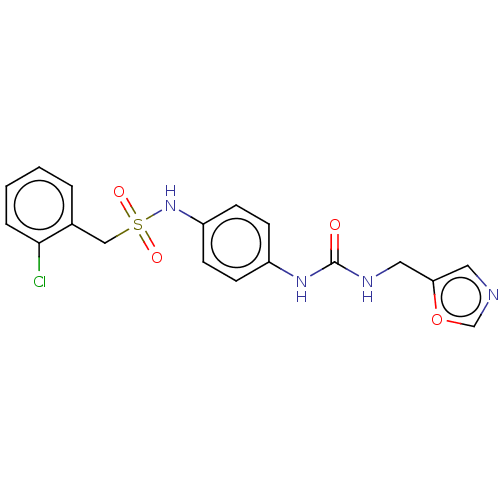 (1-(2-Chlorophenyl)-N-(4-(3-(oxazol-5-ylmethyl)urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM572964 (1-(4-((3-Chlorophenyl)sulfonyl)phenyl)-3-(oxazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | <100 | n/a | n/a | n/a | n/a |
TBA | Assay Description NAMPT reaction was performed in 50 mM HEPES, pH 7.5, containing 5 mM MgCl2, 0.1% Prionex, 0.005% Tween 20, and 1 mM TCEP at room temperature in Grein... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M048P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 653 total ) | Next | Last >> |