 Found 617 hits of ec50 data for polymerid = 50000270
Found 617 hits of ec50 data for polymerid = 50000270 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032219
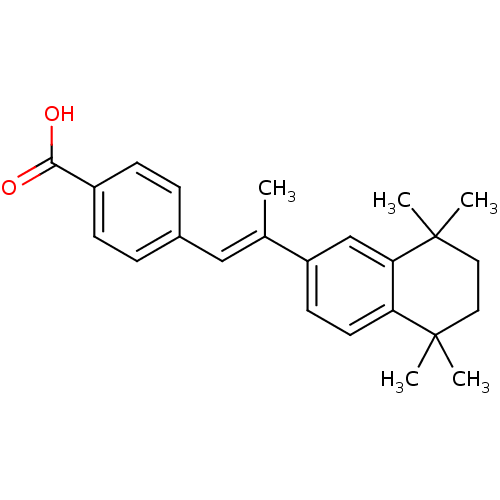
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31883
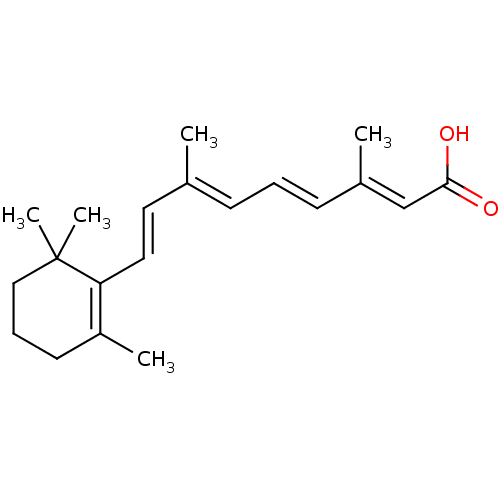
(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032224

(3-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1cccc(c1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H30O2/c1-16(12-18-8-7-9-19(14-18)23(26)27)20-15-22-21(13-17(20)2)24(3,4)10-11-25(22,5)6/h7-9,12-15H,10-11H2,1-6H3,(H,26,27)/b16-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >0.00100 | n/a | n/a | n/a | n/a |
Allergan, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR alpha |
J Med Chem 38: 2820-9 (1995)
BindingDB Entry DOI: 10.7270/Q2FN157S |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50101445
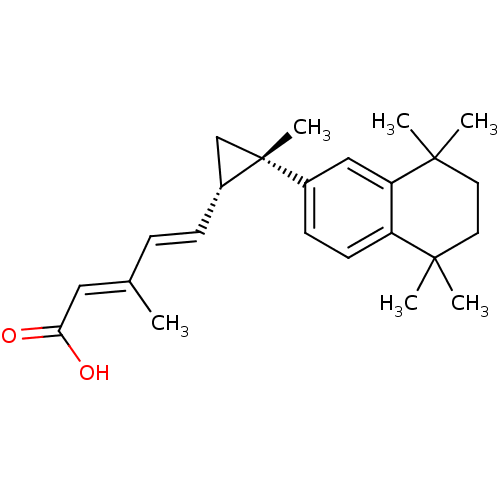
((2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@H]1C[C@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212274
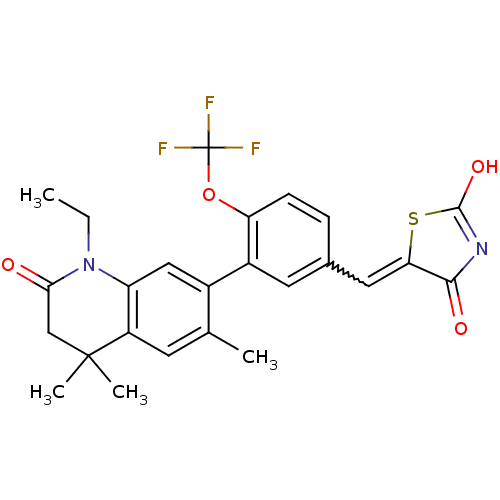
(5-((3-(1-ethyl-4,4,6-trimethyl-2-oxo-1,2,3,4-tetra...)Show SMILES CCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(C=C2SC(O)=NC2=O)ccc1OC(F)(F)F |w:19.20,c:25| Show InChI InChI=1S/C25H23F3N2O4S/c1-5-30-18-11-15(13(2)8-17(18)24(3,4)12-21(30)31)16-9-14(6-7-19(16)34-25(26,27)28)10-20-22(32)29-23(33)35-20/h6-11H,5,12H2,1-4H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212274

(5-((3-(1-ethyl-4,4,6-trimethyl-2-oxo-1,2,3,4-tetra...)Show SMILES CCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(C=C2SC(O)=NC2=O)ccc1OC(F)(F)F |w:19.20,c:25| Show InChI InChI=1S/C25H23F3N2O4S/c1-5-30-18-11-15(13(2)8-17(18)24(3,4)12-21(30)31)16-9-14(6-7-19(16)34-25(26,27)28)10-20-22(32)29-23(33)35-20/h6-11H,5,12H2,1-4H3,(H,29,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3497-503 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.047
BindingDB Entry DOI: 10.7270/Q2FQ9W93 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212417

(3-(4-(trifluoromethoxy)-3-(4,4,6-trimethyl-2-oxo-1...)Show SMILES Cc1cc2c(cc1-c1cc(CCC(O)=O)ccc1OC(F)(F)F)N(CC(F)(F)F)C(=O)CC2(C)C Show InChI InChI=1S/C24H23F6NO4/c1-13-8-17-18(31(12-23(25,26)27)20(32)11-22(17,2)3)10-15(13)16-9-14(5-7-21(33)34)4-6-19(16)35-24(28,29)30/h4,6,8-10H,5,7,11-12H2,1-3H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892
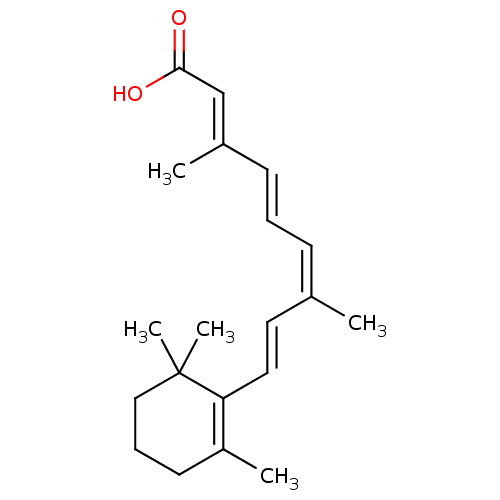
(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50101444
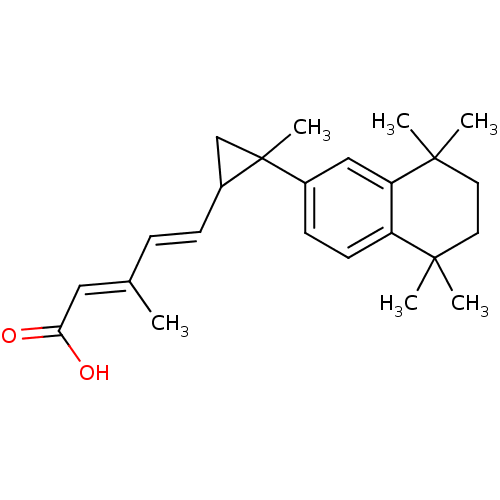
((E)-3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,...)Show SMILES C\C(\C=C\C1CC1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for functional activity in CV-1 cells transfected with an expression vector for retinoid X receptor alpha using transactivation a... |
Bioorg Med Chem Lett 6: 213-218 (1996)
Article DOI: 10.1016/0960-894X(95)00588-K
BindingDB Entry DOI: 10.7270/Q2BC3ZHZ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212277
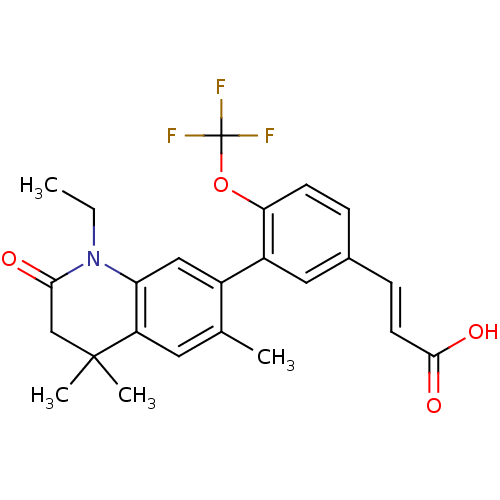
((E)-3-(3-(1-ethyl-4,4,6-trimethyl-2-oxo-1,2,3,4-te...)Show SMILES CCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(\C=C\C(O)=O)ccc1OC(F)(F)F Show InChI InChI=1S/C24H24F3NO4/c1-5-28-19-12-16(14(2)10-18(19)23(3,4)13-21(28)29)17-11-15(7-9-22(30)31)6-8-20(17)32-24(25,26)27/h6-12H,5,13H2,1-4H3,(H,30,31)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3497-503 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.047
BindingDB Entry DOI: 10.7270/Q2FQ9W93 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212277

((E)-3-(3-(1-ethyl-4,4,6-trimethyl-2-oxo-1,2,3,4-te...)Show SMILES CCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(\C=C\C(O)=O)ccc1OC(F)(F)F Show InChI InChI=1S/C24H24F3NO4/c1-5-28-19-12-16(14(2)10-18(19)23(3,4)13-21(28)29)17-11-15(7-9-22(30)31)6-8-20(17)32-24(25,26)27/h6-12H,5,13H2,1-4H3,(H,30,31)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212275
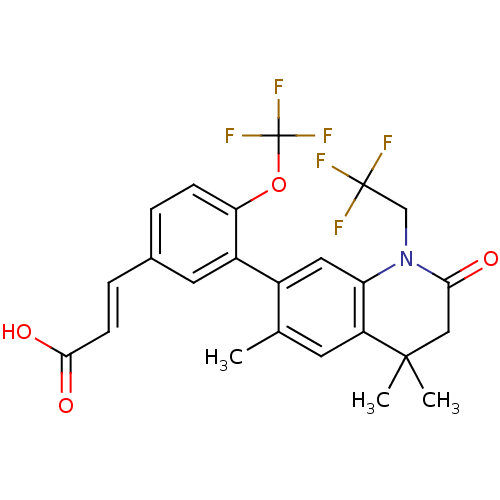
(3-(4-(trifluoromethoxy)-3-(4,4,6-trimethyl-2-oxo-1...)Show SMILES Cc1cc2c(cc1-c1cc(\C=C\C(O)=O)ccc1OC(F)(F)F)N(CC(F)(F)F)C(=O)CC2(C)C Show InChI InChI=1S/C24H21F6NO4/c1-13-8-17-18(31(12-23(25,26)27)20(32)11-22(17,2)3)10-15(13)16-9-14(5-7-21(33)34)4-6-19(16)35-24(28,29)30/h4-10H,11-12H2,1-3H3,(H,33,34)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3497-503 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.047
BindingDB Entry DOI: 10.7270/Q2FQ9W93 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129726
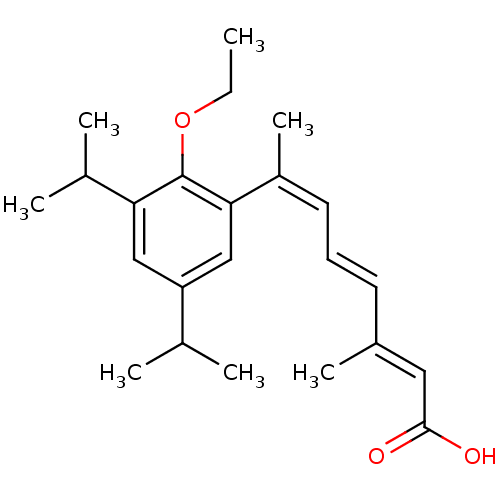
((2E,4E,6Z)-7-(2-Ethoxy-3,5-diisopropyl-phenyl)-3-m...)Show SMILES CCOc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C23H32O3/c1-8-26-23-20(16(4)5)13-19(15(2)3)14-21(23)18(7)11-9-10-17(6)12-22(24)25/h9-16H,8H2,1-7H3,(H,24,25)/b10-9+,17-12+,18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Retinoic acid receptor RXR-alpha agonist activity in vitro |
J Med Chem 46: 2683-96 (2003)
Article DOI: 10.1021/jm020340q
BindingDB Entry DOI: 10.7270/Q27H1HZ3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129726

((2E,4E,6Z)-7-(2-Ethoxy-3,5-diisopropyl-phenyl)-3-m...)Show SMILES CCOc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C23H32O3/c1-8-26-23-20(16(4)5)13-19(15(2)3)14-21(23)18(7)11-9-10-17(6)12-22(24)25/h9-16H,8H2,1-7H3,(H,24,25)/b10-9+,17-12+,18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration required for agonistic activity in CV-1 cells expressing RXR-alpha |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129717
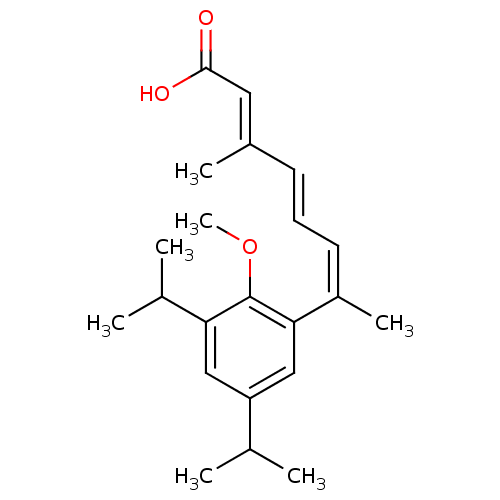
(7-(3,5-Diisopropyl-2-methoxy-phenyl)-3-methyl-octa...)Show SMILES COc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C22H30O3/c1-14(2)18-12-19(15(3)4)22(25-7)20(13-18)17(6)10-8-9-16(5)11-21(23)24/h8-15H,1-7H3,(H,23,24)/b9-8+,16-11+,17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Retinoic acid receptor RXR-alpha agonist activity in vitro |
J Med Chem 46: 2683-96 (2003)
Article DOI: 10.1021/jm020340q
BindingDB Entry DOI: 10.7270/Q27H1HZ3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052589
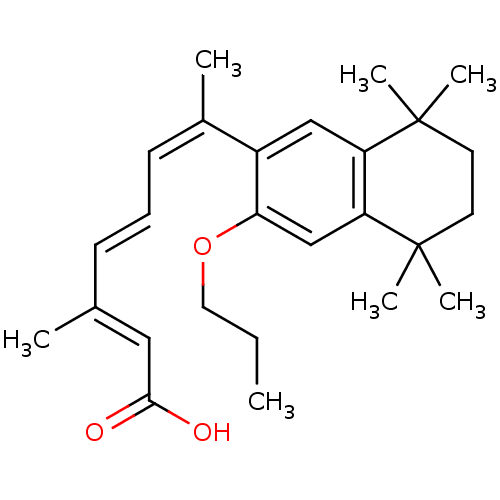
((2E,4E,6Z)-3-Methyl-7-(5,5,8,8-tetramethyl-3-propo...)Show SMILES CCCOc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional ativation of Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212266
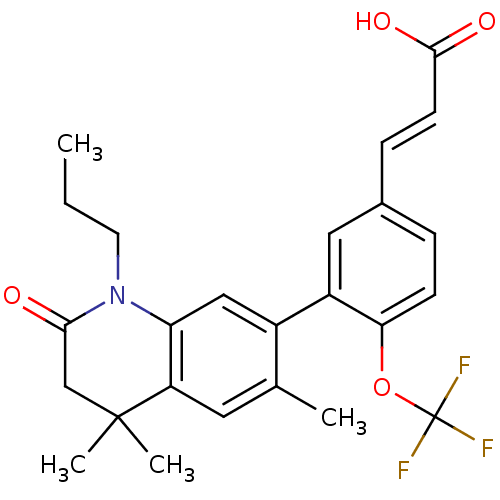
(3-(4-(trifluoromethoxy)-3-(4,4,6-trimethyl-2-oxo-1...)Show SMILES CCCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(\C=C\C(O)=O)ccc1OC(F)(F)F Show InChI InChI=1S/C25H26F3NO4/c1-5-10-29-20-13-17(15(2)11-19(20)24(3,4)14-22(29)30)18-12-16(7-9-23(31)32)6-8-21(18)33-25(26,27)28/h6-9,11-13H,5,10,14H2,1-4H3,(H,31,32)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3497-503 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.047
BindingDB Entry DOI: 10.7270/Q2FQ9W93 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212403
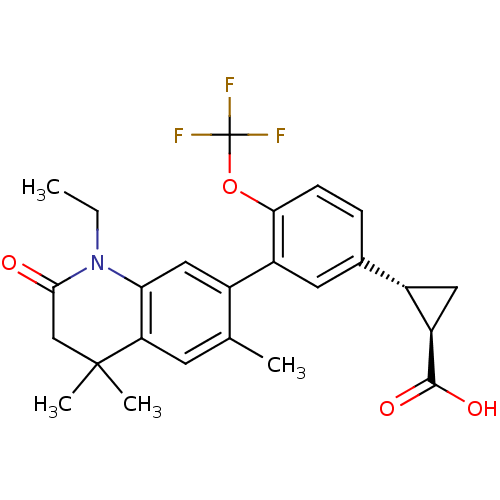
((1R,2R)-2-(3-(1-ethyl-4,4,6-trimethyl-2-oxo-1,2,3,...)Show SMILES CCN1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(ccc1OC(F)(F)F)[C@@H]1C[C@H]1C(O)=O Show InChI InChI=1S/C25H26F3NO4/c1-5-29-20-11-15(13(2)8-19(20)24(3,4)12-22(29)30)17-9-14(16-10-18(16)23(31)32)6-7-21(17)33-25(26,27)28/h6-9,11,16,18H,5,10,12H2,1-4H3,(H,31,32)/t16-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Agonist activity at human RXRalpha LBD by cell based luciferase reporter gene assay |
J Med Chem 54: 788-808 (2012)
Article DOI: 10.1021/jm101063h
BindingDB Entry DOI: 10.7270/Q208668Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826
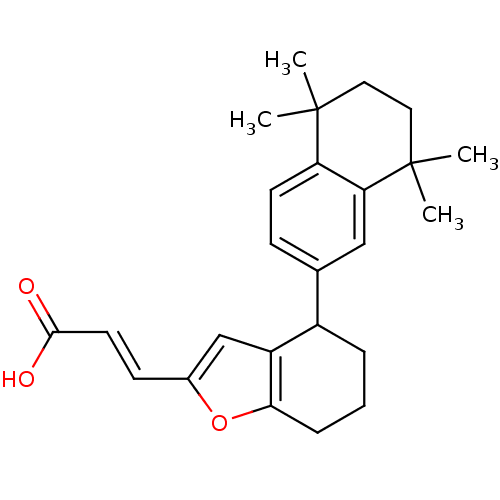
((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for Retinoid X receptor alpha activity in CV-1 cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for Retinoid X receptor alpha activity in CV-1 cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132579

((2E,4E,6Z)-7-(2-Butoxy-3,5-di-tert-butyl-phenyl)-3...)Show SMILES CCCCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(\C)=C/C=C/C(/C)=C/C(O)=O Show InChI InChI=1S/C27H40O3/c1-10-11-15-30-25-22(20(3)14-12-13-19(2)16-24(28)29)17-21(26(4,5)6)18-23(25)27(7,8)9/h12-14,16-18H,10-11,15H2,1-9H3,(H,28,29)/b13-12+,19-16+,20-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against Retinoic acid receptor RXR-alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212406
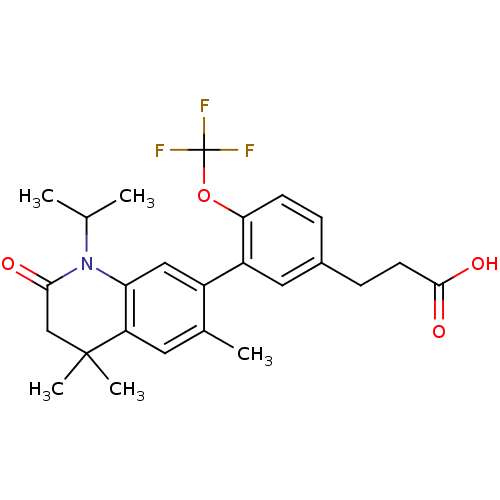
(3-(3-(1-isopropyl-4,4,6-trimethyl-2-oxo-1,2,3,4-te...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(CCC(O)=O)ccc1OC(F)(F)F Show InChI InChI=1S/C25H28F3NO4/c1-14(2)29-20-12-17(15(3)10-19(20)24(4,5)13-22(29)30)18-11-16(7-9-23(31)32)6-8-21(18)33-25(26,27)28/h6,8,10-12,14H,7,9,13H2,1-5H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132584
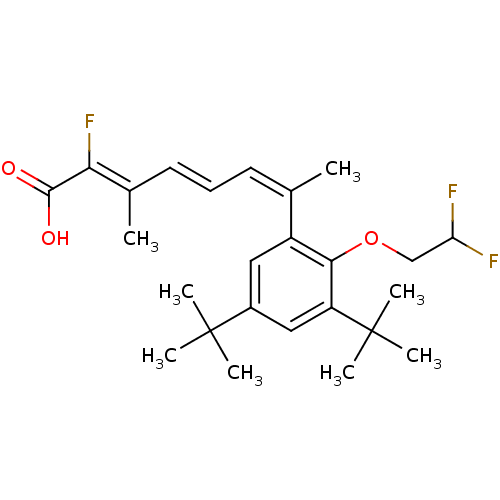
((2Z,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C(\F)C(O)=O Show InChI InChI=1S/C25H33F3O3/c1-15(10-9-11-16(2)21(28)23(29)30)18-12-17(24(3,4)5)13-19(25(6,7)8)22(18)31-14-20(26)27/h9-13,20H,14H2,1-8H3,(H,29,30)/b11-9+,15-10-,21-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against Retinoic acid receptor RXR-alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50324896
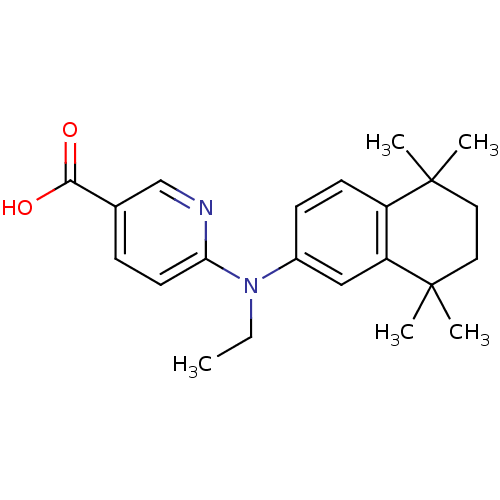
(6-[N-Ethyl(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H28N2O2/c1-6-24(19-10-7-15(14-23-19)20(25)26)16-8-9-17-18(13-16)22(4,5)12-11-21(17,2)3/h7-10,13-14H,6,11-12H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Partial agonist activity at RXRalpha (unknown origin) expressed in COS1 cells after 18 hrs by luciferase reporter gene assay |
J Med Chem 56: 1865-77 (2013)
Article DOI: 10.1021/jm400033f
BindingDB Entry DOI: 10.7270/Q2348MR8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human RXR-alpha |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01126
BindingDB Entry DOI: 10.7270/Q2DF6VVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143825
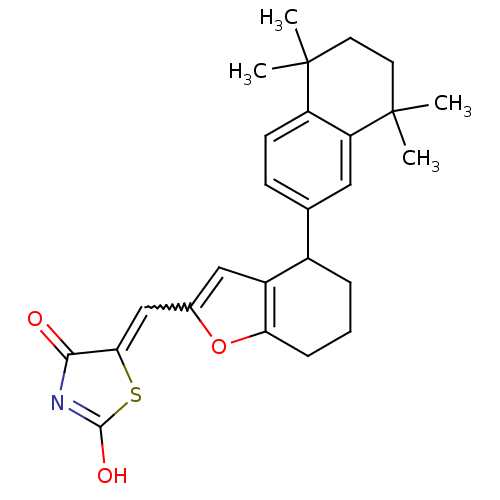
(5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(C=C3SC(O)=NC3=O)cc12 |w:21.22,c:27| Show InChI InChI=1S/C26H29NO3S/c1-25(2)10-11-26(3,4)20-12-15(8-9-19(20)25)17-6-5-7-21-18(17)13-16(30-21)14-22-23(28)27-24(29)31-22/h8-9,12-14,17H,5-7,10-11H2,1-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for Retinoid X receptor alpha activity in CV-1 cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50101446
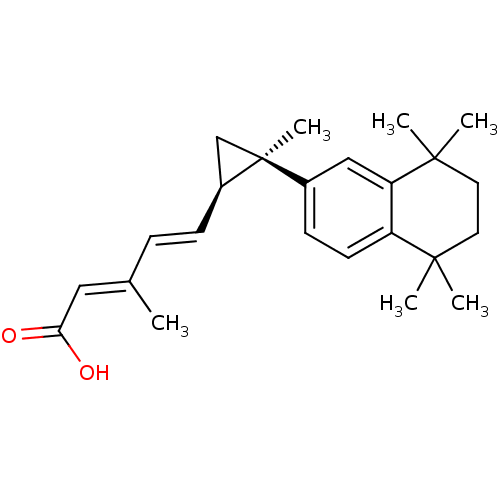
((2E,4E)-3-Methyl-5-[(1R,2R)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@H]1C[C@@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50285919
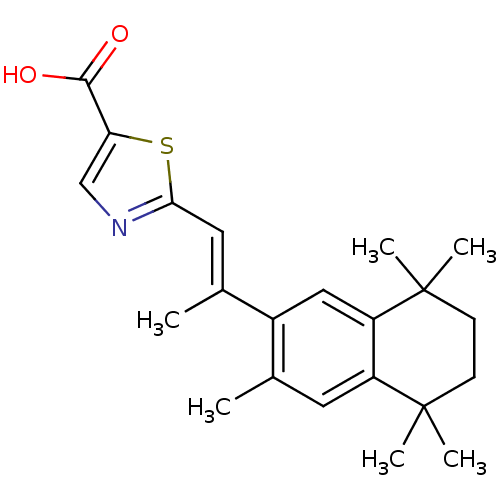
(2-[(E)-2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...)Show SMILES C\C(=C/c1ncc(s1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C22H27NO2S/c1-13-9-16-17(22(5,6)8-7-21(16,3)4)11-15(13)14(2)10-19-23-12-18(26-19)20(24)25/h9-12H,7-8H2,1-6H3,(H,24,25)/b14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity for retinoic acid receptor RXR alpha in transcriptional activation assay |
Bioorg Med Chem Lett 5: 2729-2734 (1995)
Article DOI: 10.1016/0960-894X(95)00455-3
BindingDB Entry DOI: 10.7270/Q22N527N |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290192
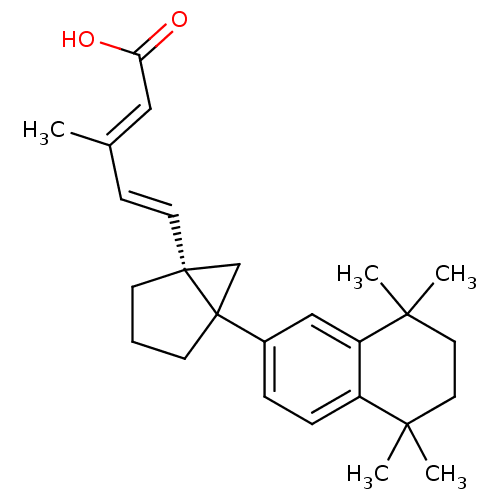
(3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...)Show SMILES C\C(\C=C\[C@]12CC1(CCC2)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C26H34O2/c1-18(15-22(27)28)9-12-25-10-6-11-26(25,17-25)19-7-8-20-21(16-19)24(4,5)14-13-23(20,2)3/h7-9,12,15-16H,6,10-11,13-14,17H2,1-5H3,(H,27,28)/b12-9+,18-15+/t25-,26?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR beta |
Bioorg Med Chem Lett 7: 2393-2398 (1997)
Article DOI: 10.1016/S0960-894X(97)00437-X
BindingDB Entry DOI: 10.7270/Q2FB52XX |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032671
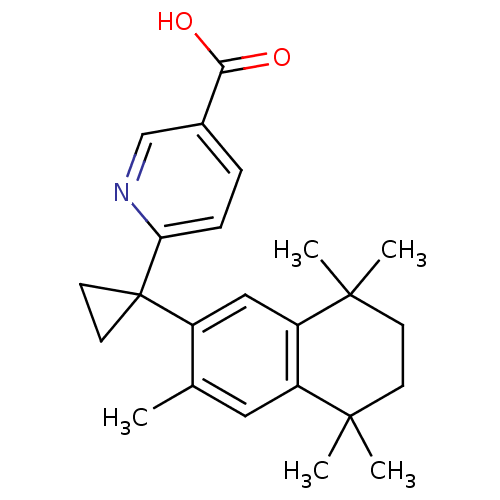
(6-[1-(3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRONAPH...)Show SMILES Cc1cc2c(cc1C1(CC1)c1ccc(cn1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO2/c1-15-12-18-19(23(4,5)9-8-22(18,2)3)13-17(15)24(10-11-24)20-7-6-16(14-25-20)21(26)27/h6-7,12-14H,8-11H2,1-5H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Ability to activate gene expression at Retinoic acid receptor RXR-alpha was evaluated in a cotransfection assay. |
J Med Chem 38: 3146-55 (1995)
BindingDB Entry DOI: 10.7270/Q2542MMT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129725
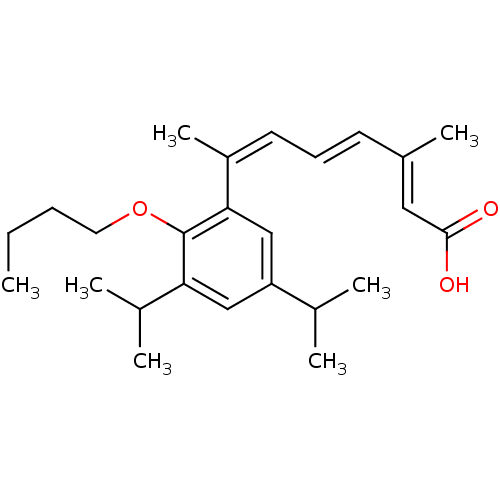
((2E,4E,6Z)-7-(2-Butoxy-3,5-diisopropyl-phenyl)-3-m...)Show SMILES CCCCOc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C25H36O3/c1-8-9-13-28-25-22(18(4)5)15-21(17(2)3)16-23(25)20(7)12-10-11-19(6)14-24(26)27/h10-12,14-18H,8-9,13H2,1-7H3,(H,26,27)/b11-10+,19-14+,20-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50212427
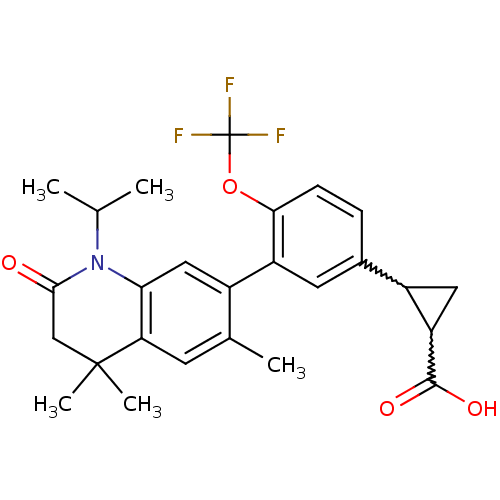
(2-(3-(1-isopropyl-4,4,6-trimethyl-2-oxo-1,2,3,4-te...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2cc(C)c(cc12)-c1cc(ccc1OC(F)(F)F)C1CC1C(O)=O |w:28.30,30.34| Show InChI InChI=1S/C26H28F3NO4/c1-13(2)30-21-11-16(14(3)8-20(21)25(4,5)12-23(30)31)18-9-15(17-10-19(17)24(32)33)6-7-22(18)34-26(27,28)29/h6-9,11,13,17,19H,10,12H2,1-5H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Activity at RXRalpha by GAL4DNA cotransfection assay |
Bioorg Med Chem Lett 17: 3491-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.049
BindingDB Entry DOI: 10.7270/Q2T153B1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074295
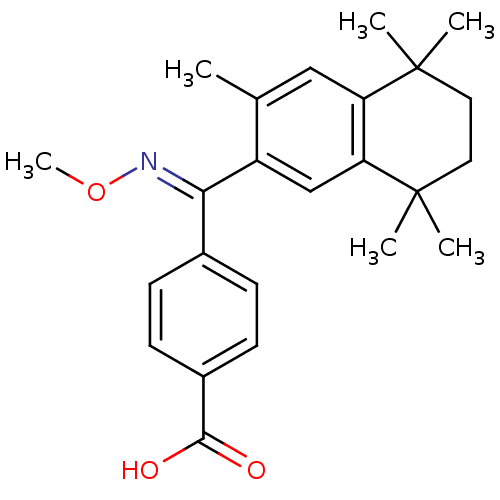
(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alpha |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143825

(5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(C=C3SC(O)=NC3=O)cc12 |w:21.22,c:27| Show InChI InChI=1S/C26H29NO3S/c1-25(2)10-11-26(3,4)20-12-15(8-9-19(20)25)17-6-5-7-21-18(17)13-16(30-21)14-22-23(28)27-24(29)31-22/h8-9,12-14,17H,5-7,10-11H2,1-4H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50143826

((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1CCCc2oc(\C=C\C(O)=O)cc12 Show InChI InChI=1S/C25H30O3/c1-24(2)12-13-25(3,4)21-14-16(8-10-20(21)24)18-6-5-7-22-19(18)15-17(28-22)9-11-23(26)27/h8-11,14-15,18H,5-7,12-13H2,1-4H3,(H,26,27)/b11-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Effective concentration for Retinoid X receptor alpha activity in CV-1 cells |
J Med Chem 47: 2010-29 (2004)
Article DOI: 10.1021/jm030565g
BindingDB Entry DOI: 10.7270/Q2KH0MRC |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074299
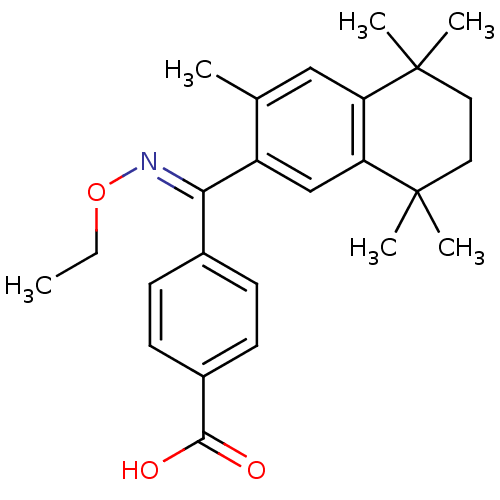
(4-[[(E)-Ethoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7,...)Show SMILES CCO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H31NO3/c1-7-29-26-22(17-8-10-18(11-9-17)23(27)28)19-15-21-20(14-16(19)2)24(3,4)12-13-25(21,5)6/h8-11,14-15H,7,12-13H2,1-6H3,(H,27,28)/b26-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alpha |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50290660
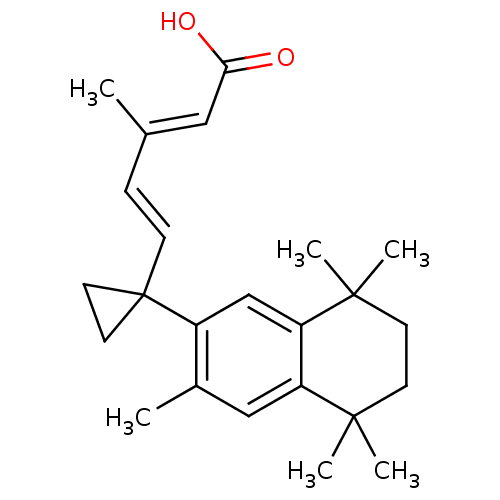
((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(CC1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-24(11-12-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 7: 2747-2752 (1997)
Article DOI: 10.1016/S0960-894X(97)10079-8
BindingDB Entry DOI: 10.7270/Q2JW8DW6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50052588
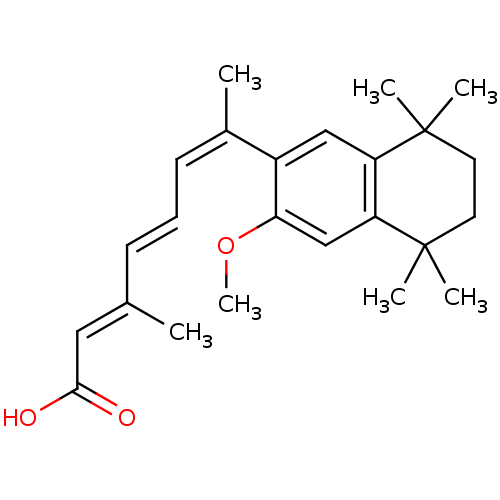
((2E,4E,6Z)-7-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,...)Show SMILES COc1cc2c(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H32O3/c1-16(13-22(25)26)9-8-10-17(2)18-14-19-20(15-21(18)27-7)24(5,6)12-11-23(19,3)4/h8-10,13-15H,11-12H2,1-7H3,(H,25,26)/b9-8+,16-13+,17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Transcriptional ativation of Retinoid X receptor RXR alpha |
J Med Chem 39: 3229-34 (1996)
Article DOI: 10.1021/jm960311d
BindingDB Entry DOI: 10.7270/Q2ZW1K14 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50324896

(6-[N-Ethyl(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H28N2O2/c1-6-24(19-10-7-15(14-23-19)20(25)26)16-8-9-17-18(13-16)22(4,5)12-11-21(17,2)3/h7-10,13-14H,6,11-12H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.28 | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5139-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.012
BindingDB Entry DOI: 10.7270/Q20G3KB3 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50324896

(6-[N-Ethyl(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...)Show SMILES CCN(c1ccc2c(c1)C(C)(C)CCC2(C)C)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C22H28N2O2/c1-6-24(19-10-7-15(14-23-19)20(25)26)16-8-9-17-18(13-16)22(4,5)12-11-21(17,2)3/h7-10,13-14H,6,11-12H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assay |
ACS Med Chem Lett 1: 521-525 (2010)
Article DOI: 10.1021/ml100184k
BindingDB Entry DOI: 10.7270/Q28S4Q6X |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00641
BindingDB Entry DOI: 10.7270/Q2K35ZR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032666

(6-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cn1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO2/c1-14-11-18-19(23(5,6)10-9-22(18,3)4)12-17(14)15(2)20-8-7-16(13-24-20)21(25)26/h7-8,11-13H,2,9-10H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Ability to activate gene expression at Retinoic acid receptor RXR-alpha was evaluated in a cotransfection assay. |
J Med Chem 38: 3146-55 (1995)
BindingDB Entry DOI: 10.7270/Q2542MMT |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074306
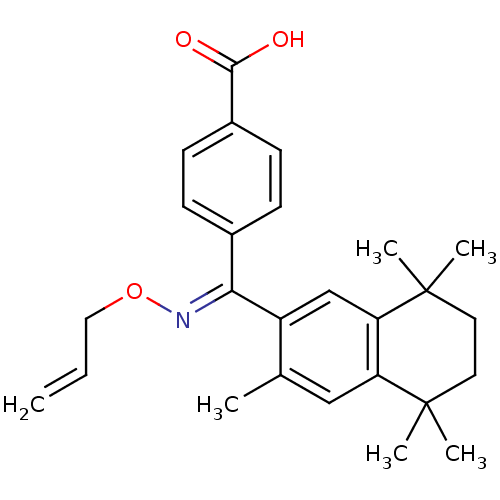
(4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...)Show SMILES Cc1cc2c(cc1\C(=N\OCC=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H31NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h7-11,15-16H,1,12-14H2,2-6H3,(H,28,29)/b27-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alpha |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50157942
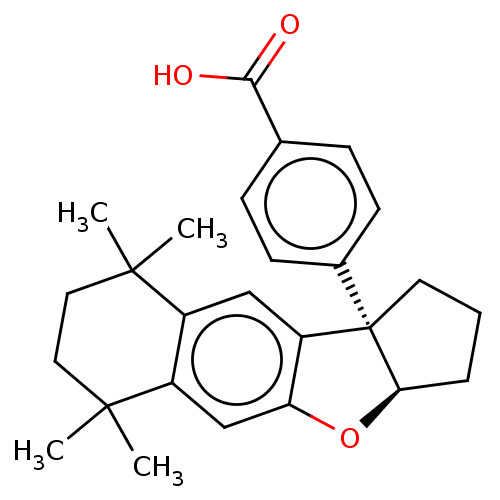
(CHEMBL3781132)Show SMILES [H][C@@]12CCC[C@@]1(c1cc3c(cc1O2)C(C)(C)CCC3(C)C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C26H30O3/c1-24(2)12-13-25(3,4)19-15-21-20(14-18(19)24)26(11-5-6-22(26)29-21)17-9-7-16(8-10-17)23(27)28/h7-10,14-15,22H,5-6,11-13H2,1-4H3,(H,27,28)/t22-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Agonist activity at histidine-tagged ligand binding domain of human RXRalpha expressed in Escherichia coli BL21 (DE3) by luciferase reporter gene ass... |
J Med Chem 59: 1232-8 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01702
BindingDB Entry DOI: 10.7270/Q2X63PT7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50157942

(CHEMBL3781132)Show SMILES [H][C@@]12CCC[C@@]1(c1cc3c(cc1O2)C(C)(C)CCC3(C)C)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C26H30O3/c1-24(2)12-13-25(3,4)19-15-21-20(14-18(19)24)26(11-5-6-22(26)29-21)17-9-7-16(8-10-17)23(27)28/h7-10,14-15,22H,5-6,11-13H2,1-4H3,(H,27,28)/t22-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Agonist activity at Renilla luciferase/GFP2-tagged RXRalpha homodimer (unknown origin) expressed in HEK293T cells by BRET2 assay |
J Med Chem 59: 1232-8 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01702
BindingDB Entry DOI: 10.7270/Q2X63PT7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146328
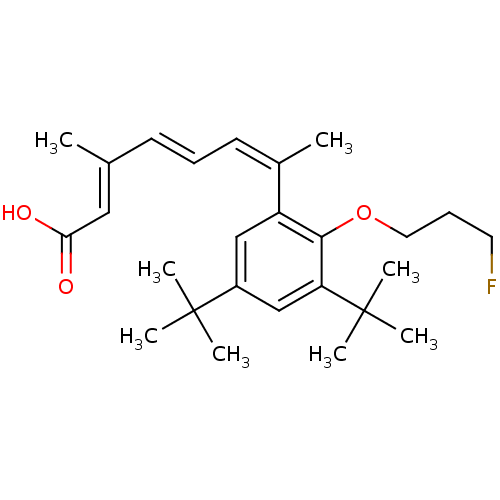
((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(3-fluoro-propox...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCCCF)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C26H37FO3/c1-18(15-23(28)29)11-9-12-19(2)21-16-20(25(3,4)5)17-22(26(6,7)8)24(21)30-14-10-13-27/h9,11-12,15-17H,10,13-14H2,1-8H3,(H,28,29)/b11-9+,18-15+,19-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data