

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421161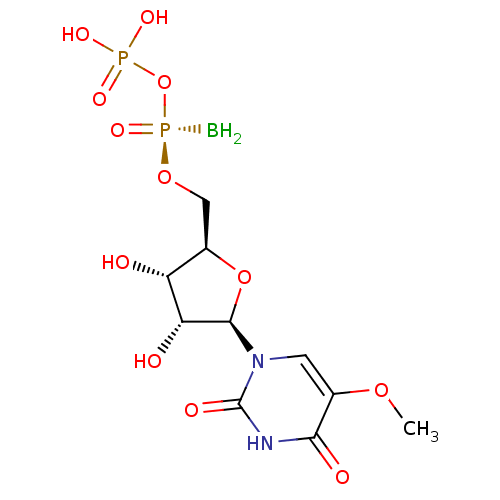 (CHEMBL2086761) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496863 (CHEMBL3220052) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496853 (CHEMBL1198754) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319139 (CHEMBL1083256 | P1-Uridine5'-P3-Cyclohexyltriphosp...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239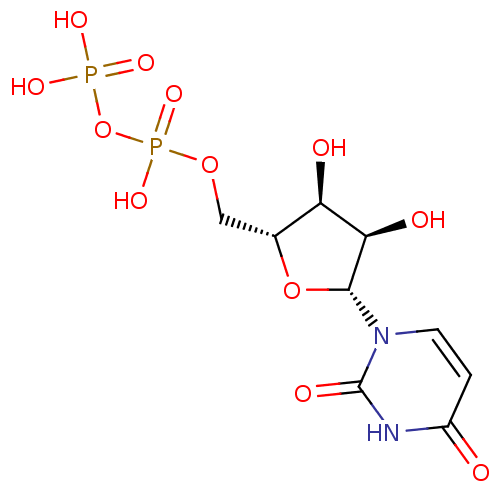 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306710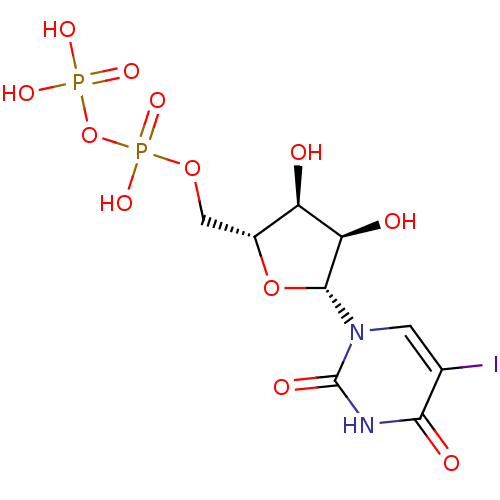 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y6 receptor expressed in human 1321N1 cells assessed as inositol phosphate accumulation | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306710 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as calcium elevation by fura2/AM assay | J Med Chem 53: 1673-85 (2010) Article DOI: 10.1021/jm901450d BindingDB Entry DOI: 10.7270/Q2ZW1MWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871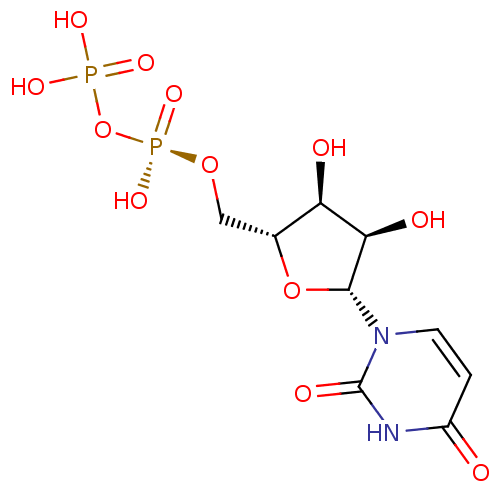 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 6 (hP2Y6) stably expressed in 131N1 astrocytoma cell | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194152 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at P2Y6 receptor expressed in human 1321N1 cells assessed as inositol phosphate accumulation | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306710 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496856 (CHEMBL3220047) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50013029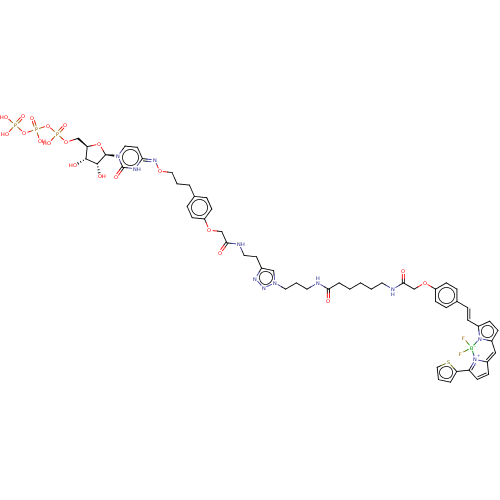 (CHEMBL3261378) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496854 (CHEMBL1198872) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421169 (CHEMBL2086770) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496855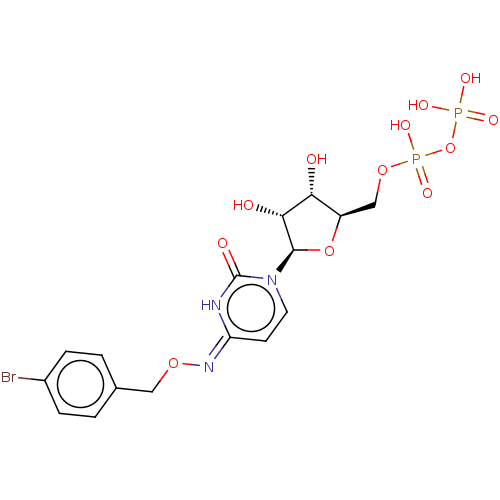 (CHEMBL3220048) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496857 (CHEMBL3220046) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496858 (CHEMBL3220050) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50303353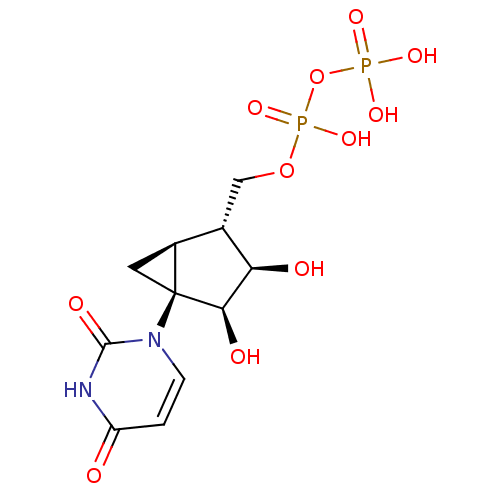 (CHEMBL1086489 | CHEMBL567321 | Triethylazanium ({[...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50303353 (CHEMBL1086489 | CHEMBL567321 | Triethylazanium ({[...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells coexpressing phospholipase C-activating Gq protein assessed as [3H]inositol p... | J Med Chem 53: 471-80 (2010) Article DOI: 10.1021/jm901432g BindingDB Entry DOI: 10.7270/Q2W95B4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306709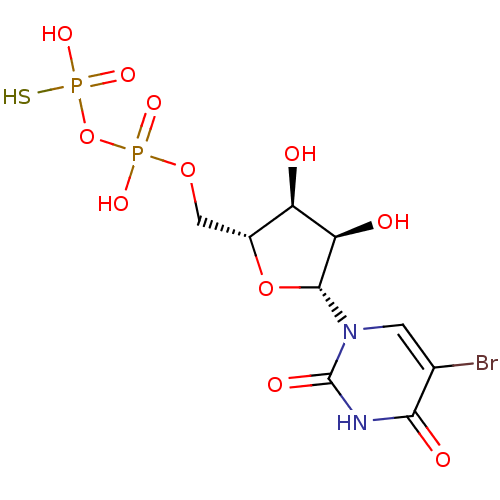 (CHEMBL602452 | O-{[(2R,3S,4R,5R)-5-(5-bromo-2,4-di...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as calcium elevation by fura2/AM assay | J Med Chem 53: 1673-85 (2010) Article DOI: 10.1021/jm901450d BindingDB Entry DOI: 10.7270/Q2ZW1MWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate intracellular accumulation by ... | J Med Chem 54: 2878-90 (2011) Article DOI: 10.1021/jm1016297 BindingDB Entry DOI: 10.7270/Q2VT1SFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306710 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-iodo-2,4-dioxo-3...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319142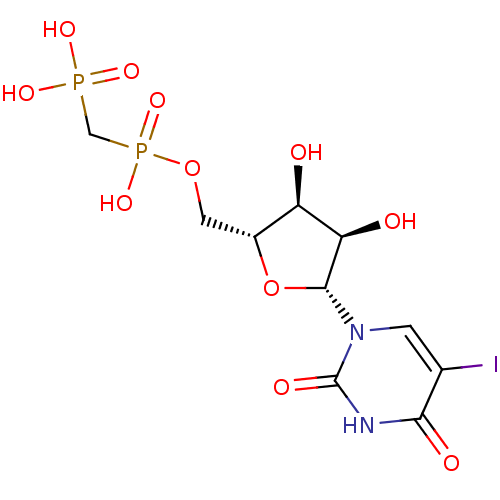 (5-Iodouridine5'-Methylenediphosphate Triethylammon...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50421165 (CHEMBL2086765) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y6 receptor expressed in human 1321N1 cells assessed as intracellular calcium mobilization by fura2/AM-based f... | Bioorg Med Chem 20: 5483-95 (2012) Article DOI: 10.1016/j.bmc.2012.07.042 BindingDB Entry DOI: 10.7270/Q21837S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194160 ((2R,3R,4S,5R)-1-(3,4-dihydroxy-5-(diphosphoryloxym...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319130 (((((2R,3S,4R,5R)-3,4-dihydroxy-5-((E)-4-(methoxyim...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199191 (((2R,3S,4R,5R)-5-(2,4-dioxo-3-(2-oxo-2-phenylethyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199191 (((2R,3S,4R,5R)-5-(2,4-dioxo-3-(2-oxo-2-phenylethyl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319129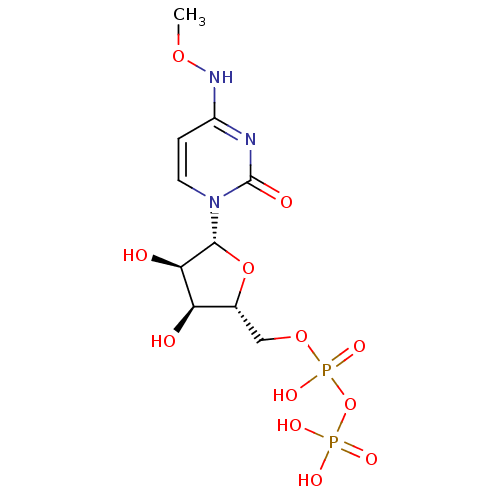 (CHEMBL1084020 | CHEMBL1204010 | N4-Methoxycytidine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells coexpressing phospholipase C-activating Gq protein assessed as [3H]inositol p... | J Med Chem 53: 471-80 (2010) Article DOI: 10.1021/jm901432g BindingDB Entry DOI: 10.7270/Q2W95B4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50306706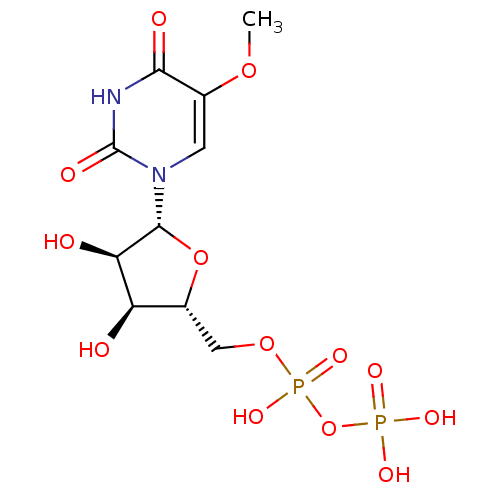 (((2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methoxy-2,4-diox...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as calcium elevation by fura2/AM assay | J Med Chem 53: 1673-85 (2010) Article DOI: 10.1021/jm901450d BindingDB Entry DOI: 10.7270/Q2ZW1MWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319143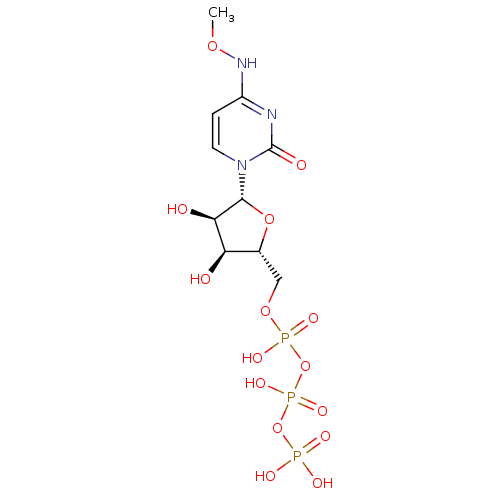 (CHEMBL1083764 | CHEMBL1198849 | N4-Methoxycytidine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50194147 (4-Thio-UDP | CHEMBL384992 | [(2R,3S,4R,5R)-3,4-dih...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in 1321N1 cells assessed as PLC-mediated [3H]IP production | J Med Chem 49: 5532-43 (2006) Article DOI: 10.1021/jm060485n BindingDB Entry DOI: 10.7270/Q2ZC83NW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50118239 (CHEMBL130266 | UDP | Uridine diphosphate | uridine...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Stimulation of phospholipase C in 1321N1 astrocytoma cells transfected with human P2Y6 receptor | J Med Chem 48: 8108-11 (2005) Article DOI: 10.1021/jm050911p BindingDB Entry DOI: 10.7270/Q28915FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50012975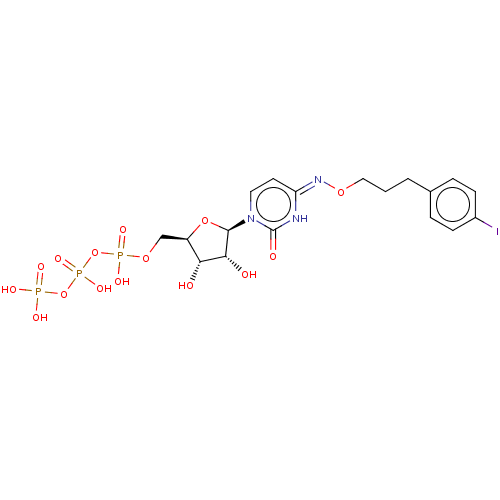 (CHEMBL3261366) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50013012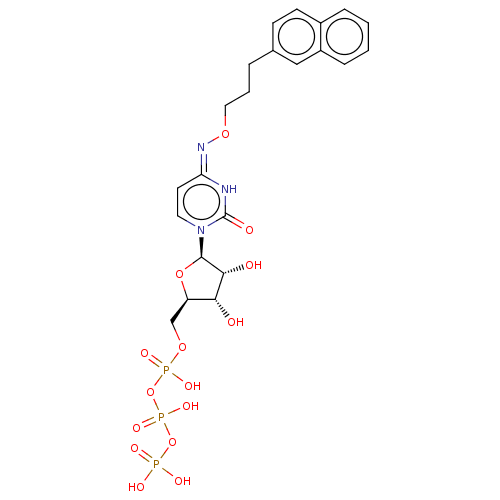 (CHEMBL3261374) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells assessed as stimulation of [3H]inositol phosphate accumulation by liquid scin... | J Med Chem 57: 3874-83 (2014) Article DOI: 10.1021/jm500367e BindingDB Entry DOI: 10.7270/Q2V989M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50523545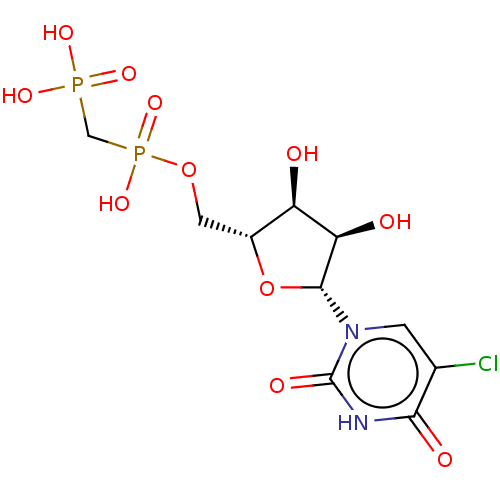 (CHEMBL4538320) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50523545 (CHEMBL4538320) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496862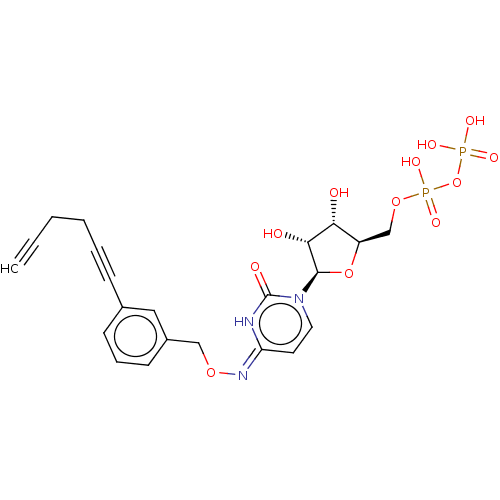 (CHEMBL3220051) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50403871 (URIDINE_DIPHOSPHATE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity evaluated as change in the level of cytosolic calcium in 1321N astrocytoma cells infected with a retrovirus encoding the human P2Y6 ... | Bioorg Med Chem Lett 11: 157-60 (2001) BindingDB Entry DOI: 10.7270/Q2BG2PH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50523532 (CHEMBL4457058) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells assessed as calcium mobilization measured by microplate reader method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50199186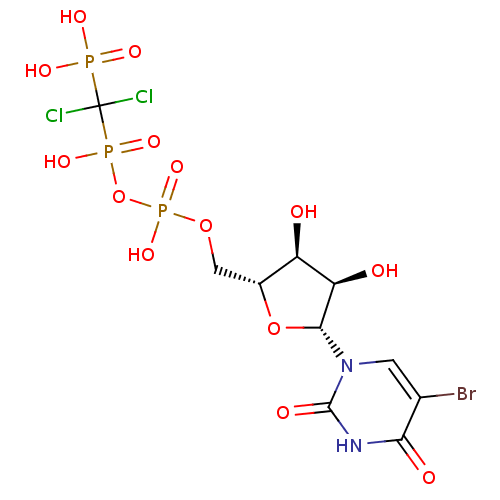 (5-bromouridine-5'-uridylic acid (1,1-dichloro-1-ph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in 1321N1 cells assessed as IP accumulation by SPA | J Med Chem 49: 7076-87 (2006) Article DOI: 10.1021/jm060848j BindingDB Entry DOI: 10.7270/Q2SN09RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496861 (CHEMBL3220054) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50303340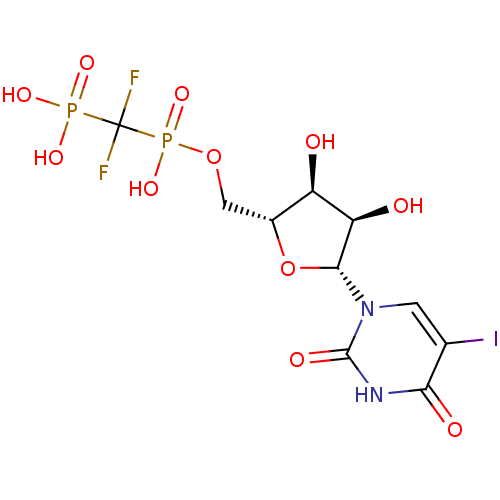 (({[(2R,3S,4R,5R)-3,4-Dihydroxy-5-(5-iodo-2,4-dioxo...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 127 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human P2Y6 receptor expressed in human 1321N1 cells coexpressing phospholipase C-activating Gq protein assessed as [3H]inositol p... | J Med Chem 53: 471-80 (2010) Article DOI: 10.1021/jm901432g BindingDB Entry DOI: 10.7270/Q2W95B4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319142 (5-Iodouridine5'-Methylenediphosphate Triethylammon...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells assessed as [3H]inositol phosphate production by scintillation pr... | J Med Chem 53: 4488-501 (2010) Article DOI: 10.1021/jm100287t BindingDB Entry DOI: 10.7270/Q2RB74SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50319142 (5-Iodouridine5'-Methylenediphosphate Triethylammon...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human P2Y6R expressed in 1321N1 cells measured after 30 mins by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128137 BindingDB Entry DOI: 10.7270/Q27P935K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 6 (Homo sapiens (Human)) | BDBM50496860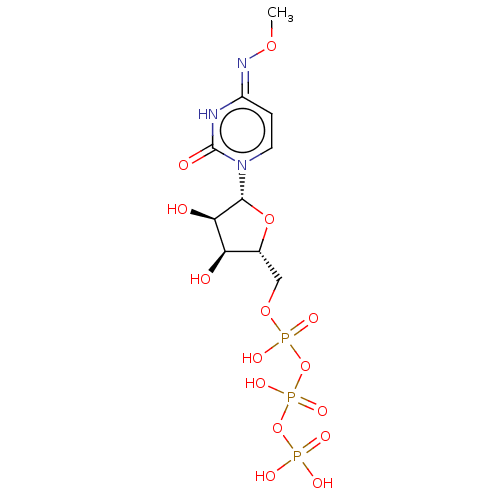 (CHEMBL1198849) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Agonist activity at human recombinant P2Y6 receptor expressed in human 1321N1 cells using [3H]inositol as substrate assessed as [3H]inositol phosphat... | Medchemcomm 4: 1156-1165 (2013) Article DOI: 10.1039/c3md00132f BindingDB Entry DOI: 10.7270/Q20G3P36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 319 total ) | Next | Last >> |