

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118217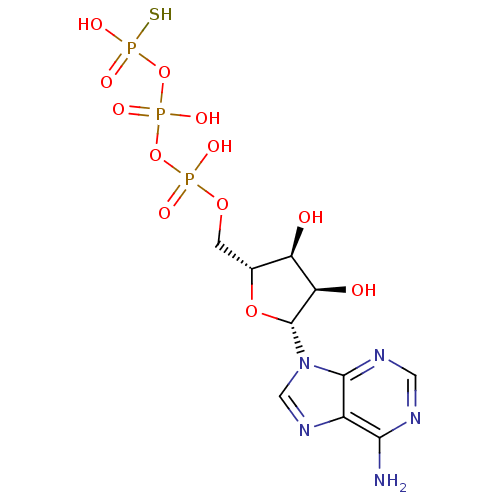 (ADENOSINE-5'-DIPHOSPHATE MONOTHIOPHOSPHATE | ATP-g...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at P2Y11 receptor expressed in 1321N1 cells assessed as cytosolic calcium level | J Med Chem 50: 5600-7 (2007) Article DOI: 10.1021/jm070043r BindingDB Entry DOI: 10.7270/Q2445Q7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435008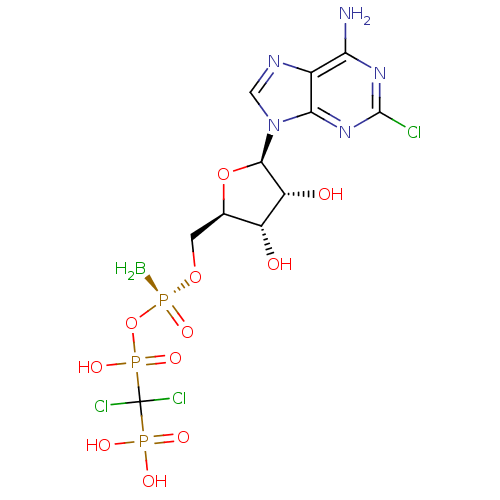 (CHEMBL2386496) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50477240 (8-CARBOXY-ISO-IANTHERAN A) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 479 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at P2Y11 receptor expressed in 1321N1 cells assessed as cytosolic calcium level | J Med Chem 50: 5600-7 (2007) Article DOI: 10.1021/jm070043r BindingDB Entry DOI: 10.7270/Q2445Q7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against Cathepsin B activity | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131061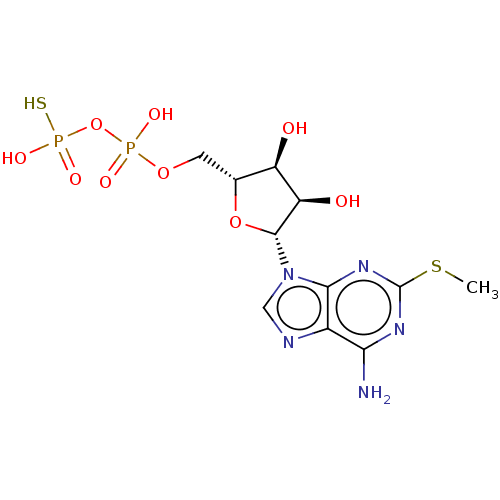 (CHEMBL3634185) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50019292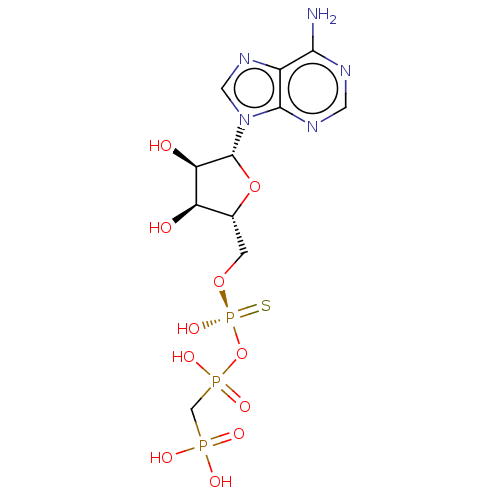 (CHEMBL3289393) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y11 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131060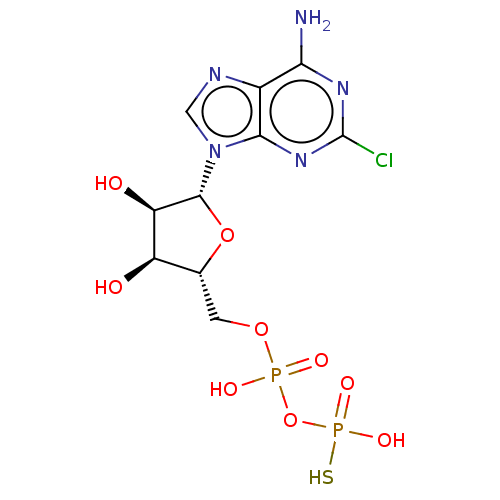 (CHEMBL3634186) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435010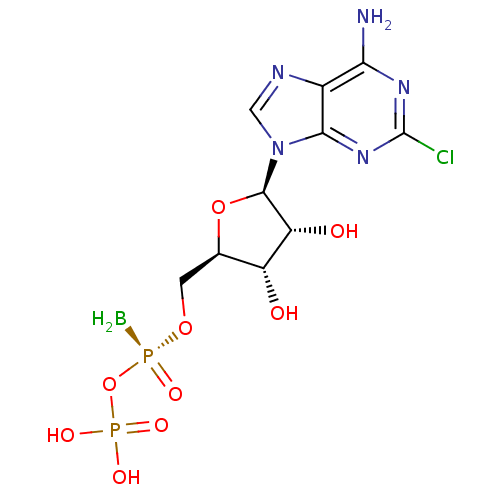 (CHEMBL2386492) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131064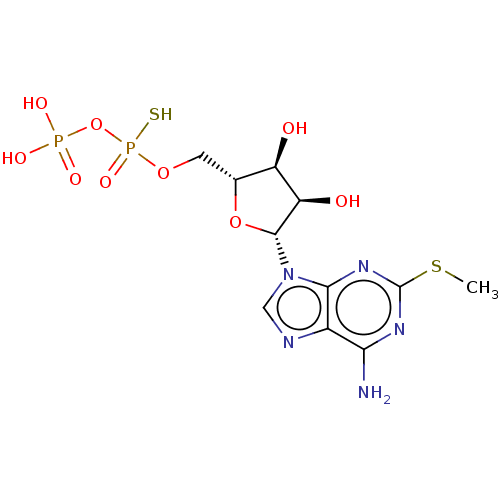 (CHEMBL3634182) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibition of [3H]-U-69,593 binding to Opioid receptor kappa 1 | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131064 (CHEMBL3634182) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131062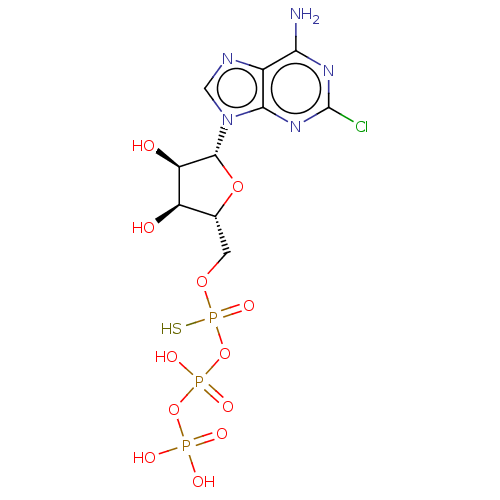 (CHEMBL3634184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131062 (CHEMBL3634184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50477239 (ISO-IANTHERAN A) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Agonist activity at P2Y11 receptor expressed in 1321N1 cells assessed as cytosolic calcium level | J Med Chem 50: 5600-7 (2007) Article DOI: 10.1021/jm070043r BindingDB Entry DOI: 10.7270/Q2445Q7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435009 (CHEMBL2386495) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118223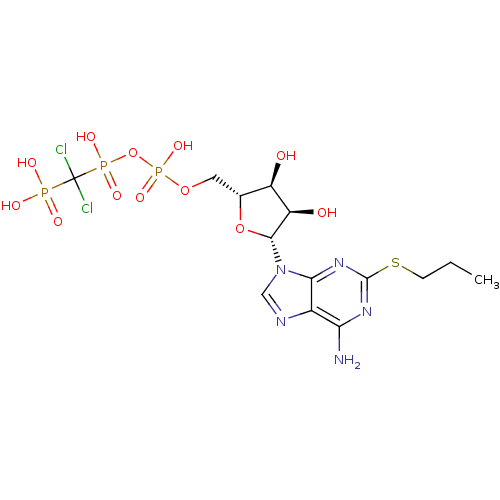 (AR-C67085 | Adenosine triphosphate derivative | CH...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435012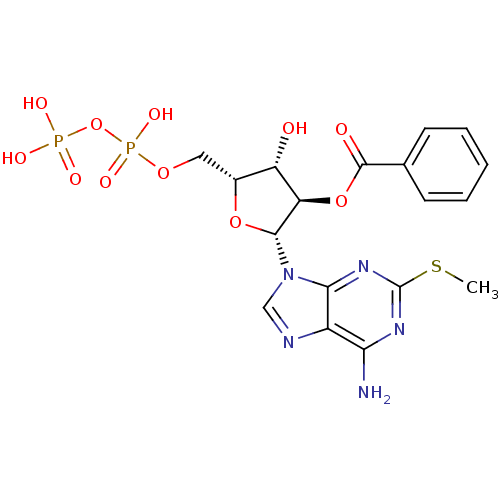 (CHEMBL2386490) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50398070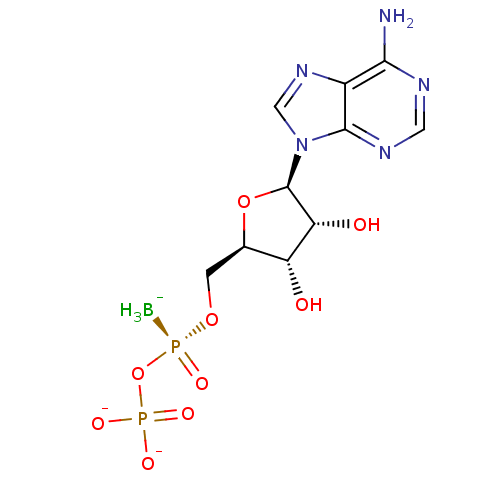 (CHEMBL2181938) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118217 (ADENOSINE-5'-DIPHOSPHATE MONOTHIOPHOSPHATE | ATP-g...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM2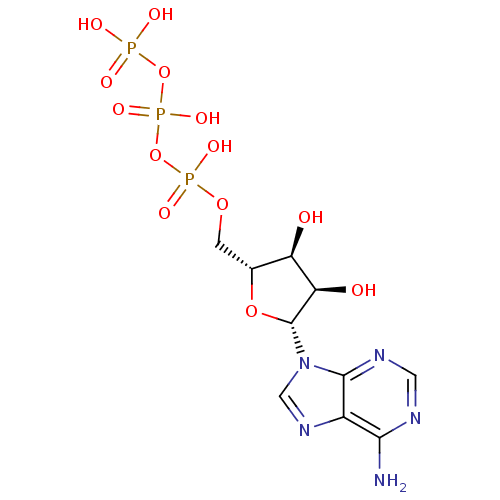 (({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP tagged-human P2Y11 receptor expressed in human 1321N1 cells assessed as elevation in calcium level after 30 mins by fluoresce... | J Med Chem 53: 8485-97 (2010) Article DOI: 10.1021/jm100597c BindingDB Entry DOI: 10.7270/Q22V2GF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50435011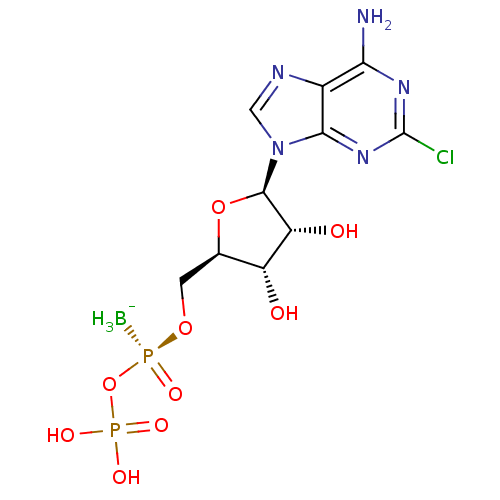 (CHEMBL2386491) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480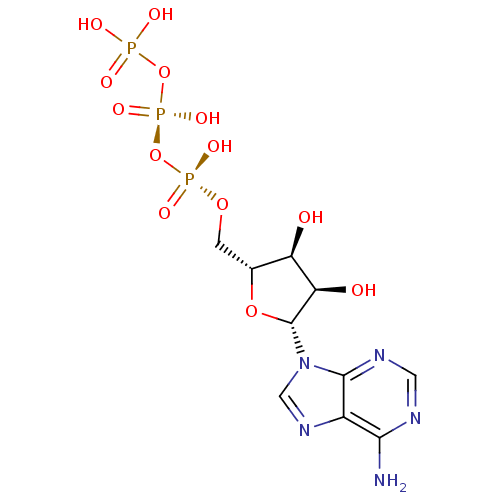 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human GFP-tagged P2Y11R transfected in human 1321N1 cells assessed as induction of intracellular calcium mobilization by fluoresc... | J Med Chem 56: 4938-52 (2013) Article DOI: 10.1021/jm400197m BindingDB Entry DOI: 10.7270/Q2Q52R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118219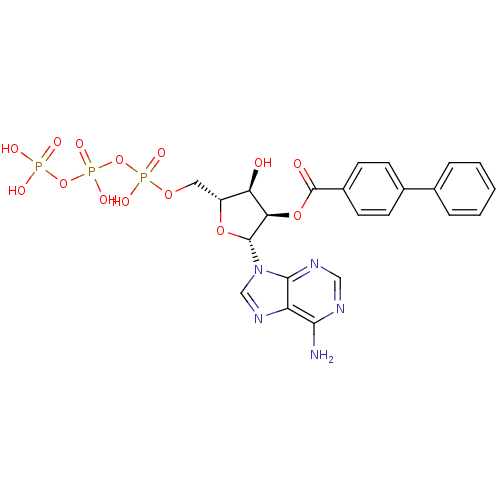 (Bz-ATP | CHEMBL339386) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131059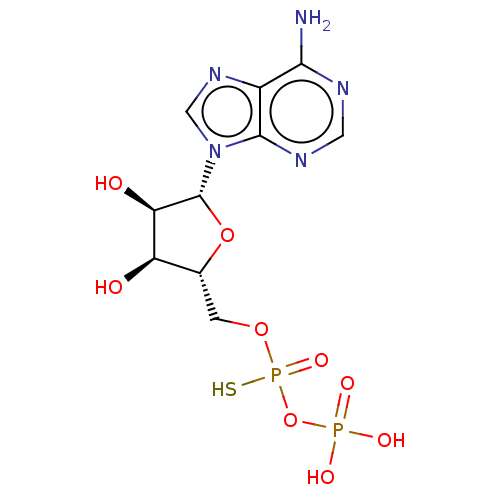 (CHEMBL575257) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131059 (CHEMBL575257) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118233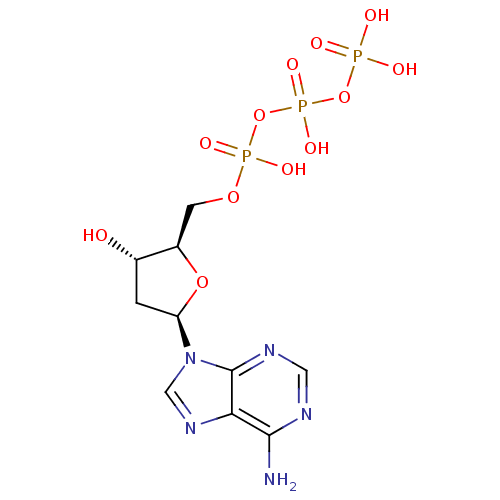 (2'-deoxyadenosine 5'-(tetrahydrogen triphosphate) ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50347445 (CHEMBL1802094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP tagged-human P2Y11 receptor expressed in human 1321N1 cells assessed as elevation in calcium level after 30 mins by fluoresce... | J Med Chem 53: 8485-97 (2010) Article DOI: 10.1021/jm100597c BindingDB Entry DOI: 10.7270/Q22V2GF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50336799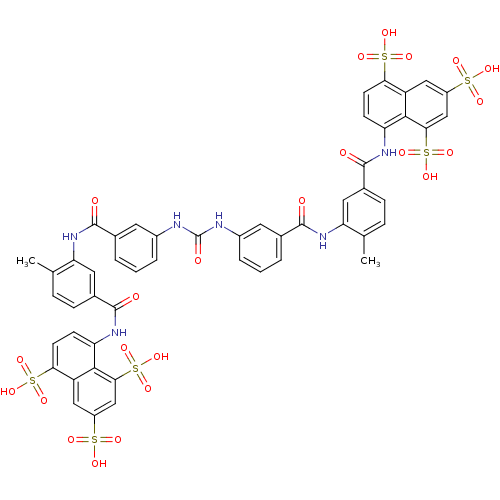 (5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM18137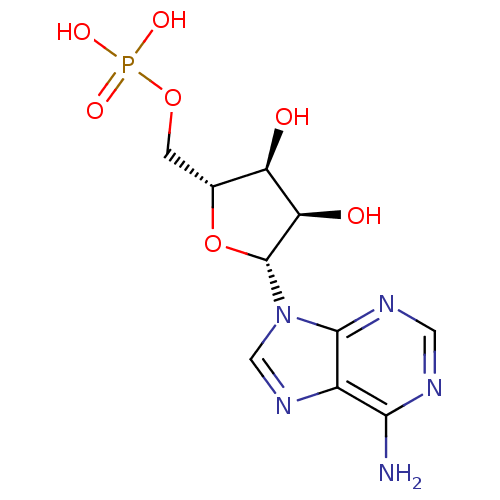 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uM | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50409486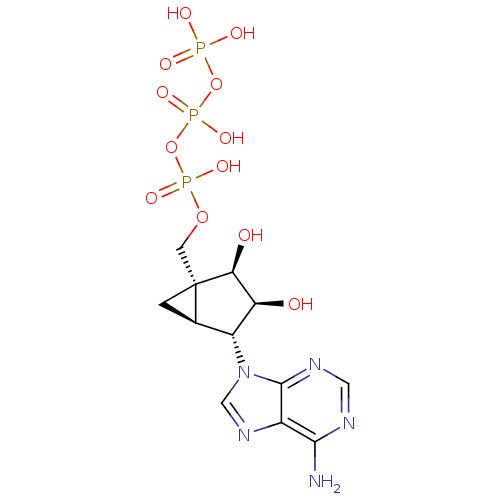 (CHEMBL2111533) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Measure of Agonist Potency at human P2Y purinoceptor 11 (hP2Y11) stably expressed in 131N1 astrocytoma cell at 10 uM | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity by measuring inositol phosphate accumulation in 1321N1 human astrocytoma cells stably expressing human P2Y purinoceptor 11 | J Med Chem 45: 2090-100 (2002) BindingDB Entry DOI: 10.7270/Q25H7GZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118230 (5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118230 (5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118232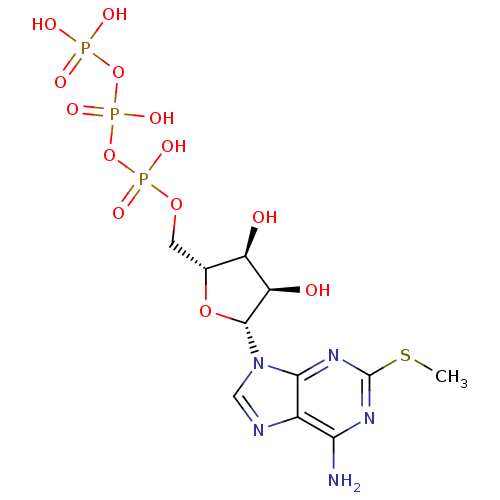 (2-MeSATP | ATP, 2-meS | CHEMBL336208) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118213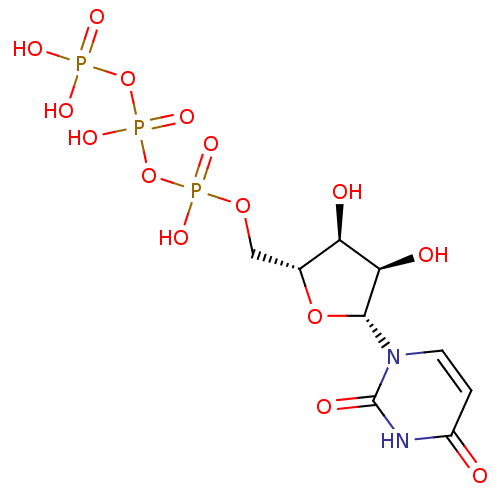 (5'-UTP | CHEMBL336296 | H4utp | UTP | uridine 5'-(...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50029031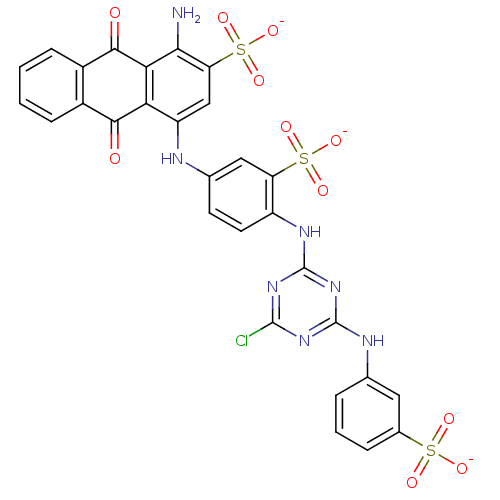 (1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50026893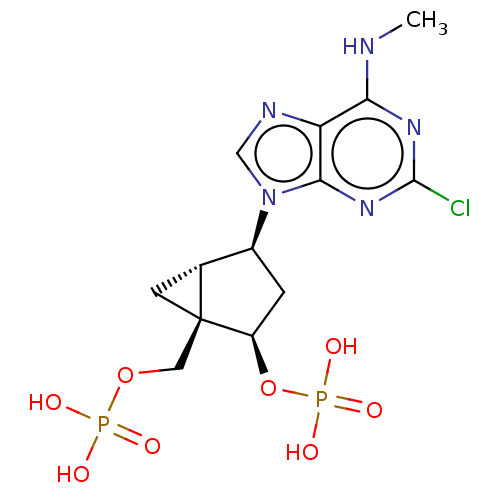 (CHEMBL3085531) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||